The Piriformospora indica effector PIIN_08944 promotes the mutualistic Sebacinalean symbiosis
- PMID: 26579156
- PMCID: PMC4620400
- DOI: 10.3389/fpls.2015.00906
The Piriformospora indica effector PIIN_08944 promotes the mutualistic Sebacinalean symbiosis
Abstract
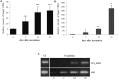
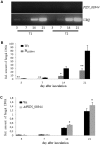
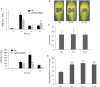
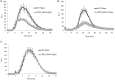
Pathogenic and mutualistic microbes actively suppress plant defense by secreting effector proteins to manipulate the host responses for their own benefit. Current knowledge about fungal effectors has been mainly derived from biotrophic and hemibiotrophic plant pathogenic fungi and oomycetes with restricted host range. We studied colonization strategies of the root endophytic basidiomycete Piriformospora indica that colonizes a wide range of plant species thereby establishing long-term mutualistic relationships. The release of P. indica's genome helped to identify hundreds of genes coding for candidate effectors and provides an opportunity to investigate the role of those proteins in a mutualistic symbiosis. We demonstrate that the candidate effector PIIN_08944 plays a crucial role during fungal colonization of Arabidopsis thaliana roots. PIIN_08944 expression was detected during chlamydospore germination, and fungal deletion mutants (PiΔ08944) showed delayed root colonization. Constitutive over-expression of PIIN_08944 in Arabidopsis rescued the delayed colonization phenotype of the deletion mutant. PIIN_08944-expressing Arabidopsis showed a reduced expression of flg22-induced marker genes of pattern-triggered immunity (PTI) and the salicylic acid (SA) defense pathway, and expression of PIIN_08944 in barley reduced the burst of reactive oxygen species (ROS) triggered by flg22 and chitin. These data suggest that PIIN_08944 contributes to root colonization by P. indica by interfering with SA-mediated basal immune responses of the host plant. Consistent with this, PIIN_08944-expressing Arabidopsis also supported the growth of the biotrophic oomycete Hyaloperonospora arabidopsidis while growth of the necrotrophic fungi Botrytis cinerea on Arabidopsis and Fusarium graminearum on barley was not affected.
Keywords: Piriformospora indica; endophyte; fungal effectors; mutualist; root; small secreted proteins; symbiosis.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

