Atypical centromeres in plants-what they can tell us
- PMID: 26579160
- PMCID: PMC4620154
- DOI: 10.3389/fpls.2015.00913
Atypical centromeres in plants-what they can tell us
Abstract
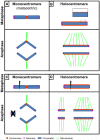
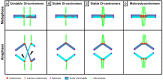
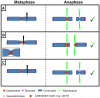
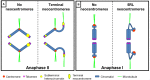
The centromere, visible as the primary constriction of condensed metaphase chromosomes, is a defined chromosomal locus essential for genome stability. It mediates transient assembly of a multi-protein complex, the kinetochore, which enables interaction with spindle fibers and thus faithful segregation of the genetic information during nuclear divisions. Centromeric DNA varies in extent and sequence composition among organisms, but a common feature of almost all active eukaryotic centromeres is the presence of the centromeric histone H3 variant cenH3 (a.k.a. CENP-A). These typical centromere features apply to most studied species. However, a number of species display "atypical" centromeres, such as holocentromeres (centromere extension along almost the entire chromatid length) or neocentromeres (ectopic centromere activity). In this review, we provide an overview of different atypical centromere types found in plants including holocentromeres, de novo formed centromeres and terminal neocentromeres as well as di-, tri- and metapolycentromeres (more than one centromere per chromosomes). We discuss their specific and common features and compare them to centromere types found in other eukaryotic species. We also highlight new insights into centromere biology gained in plants with atypical centromeres such as distinct mechanisms to define a holocentromere, specific adaptations in species with holocentromeres during meiosis or various scenarios leading to neocentromere formation.
Keywords: cenH3; centromere; holocentric chromosomes; kinetochore; meiosis; mitosis; neocentromeres; plants.
Figures
Similar articles
-
Restructuring of Holocentric Centromeres During Meiosis in the Plant Rhynchospora pubera.Genetics. 2016 Oct;204(2):555-568. doi: 10.1534/genetics.116.191213. Epub 2016 Aug 3. Genetics. 2016. PMID: 27489000 Free PMC article.
-
Holocentromere identity: from the typical mitotic linear structure to the great plasticity of meiotic holocentromeres.Chromosoma. 2016 Sep;125(4):669-81. doi: 10.1007/s00412-016-0612-7. Epub 2016 Aug 16. Chromosoma. 2016. PMID: 27530342 Review.
-
Super-Resolution Microscopy Reveals Diversity of Plant Centromere Architecture.Int J Mol Sci. 2020 May 15;21(10):3488. doi: 10.3390/ijms21103488. Int J Mol Sci. 2020. PMID: 32429054 Free PMC article. Review.
-
Evolution of holocentric chromosomes: Drivers, diversity, and deterrents.Semin Cell Dev Biol. 2022 Jul;127:90-99. doi: 10.1016/j.semcdb.2022.01.003. Epub 2022 Jan 11. Semin Cell Dev Biol. 2022. PMID: 35031207 Review.
-
Holocentromeres are dispersed point centromeres localized at transcription factor hotspots.Elife. 2014 Jan 1;3:e02025. doi: 10.7554/eLife.02025. Elife. 2014. PMID: 24714495 Free PMC article.
Cited by
-
Endopolyploidy is a common response to UV-B stress in natural plant populations, but its magnitude may be affected by chromosome type.Ann Bot. 2020 Oct 6;126(5):883-889. doi: 10.1093/aob/mcaa109. Ann Bot. 2020. PMID: 32582956 Free PMC article.
-
Holocentric chromosomes.PLoS Genet. 2020 Jul 30;16(7):e1008918. doi: 10.1371/journal.pgen.1008918. eCollection 2020 Jul. PLoS Genet. 2020. PMID: 32730246 Free PMC article.
-
The Genomics of Plant Satellite DNA.Prog Mol Subcell Biol. 2021;60:103-143. doi: 10.1007/978-3-030-74889-0_5. Prog Mol Subcell Biol. 2021. PMID: 34386874 Review.
-
Exploring Plant Meiosis: Insights from the Kinetochore Perspective.Curr Issues Mol Biol. 2023 Sep 28;45(10):7974-7995. doi: 10.3390/cimb45100504. Curr Issues Mol Biol. 2023. PMID: 37886947 Free PMC article. Review.
-
Conservation of centromeric histone 3 interaction partners in plants.J Exp Bot. 2020 Aug 17;71(17):5237-5246. doi: 10.1093/jxb/eraa214. J Exp Bot. 2020. PMID: 32369582 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

