Dysferlinopathy Fibroblasts Are Defective in Plasma Membrane Repair
- PMID: 26579332
- PMCID: PMC4639325
- DOI: 10.1371/currents.md.5865add2d766f39a0e0411d38a7ba09c
Dysferlinopathy Fibroblasts Are Defective in Plasma Membrane Repair
Abstract
Background: Dysferlin is a sarcolemmal protein that is defective in Miyoshi myopathy and limb-girdle muscular dystrophy type 2B, and is involved in sarcolemmal repair. Primary cultured myoblasts and myotubes established from patient muscle biopsies have been widely utilized to explore the molecular mechanism of dysferlinopathy.
Objectives: The purpose of this study was to explore the possible utility of dermal fibroblasts from dysferlin-deficient patients and SJL mice as a tool for studying dysferlinopathy.
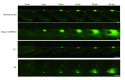
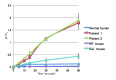
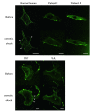
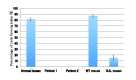
Methods: Dysferlin protein expression in fibroblasts from dysferlin-deficient patients and SJL mice was analyzed by immunoblotting and immunocytochemistry. The membrane wound-repair assay was performed on the fibroblasts using a confocal microscope equipped with a UV-laser. The membrane blebbing assay using hypotonic shock, in which normal membrane blebbing is detected only in the presence of dysferlin, was also performed using human and mouse fibroblasts.
Results: Mis-sense mutated dysferlin was expressed at a very low level in fibroblasts from a dysferlinopathy patient, and lower expression level of truncated dysferlin was observed in SJL mouse fibroblast. Fibroblasts from patients with dysferlinopathy and SJL mice showed attenuated membrane repair and did not form membrane blebs in response to hypoosmotic shock. Proteosomal inhibitior increased mis-sense mutated or truncated dysferlin levels, and restored membrane blebbing, however, proteosomal inhibition failed to improve levels of dysferlin with non-sense or frame-shift mutation.
Conclusion: Fibroblasts from dysferlinopathy patients and SJL mice showed attenuated plasma membrane repair, and could be a tool for studying dysferlinopathy.
Figures




Similar articles
-
Dysferlin and animal models for dysferlinopathy.J Toxicol Pathol. 2012 Jun;25(2):135-47. doi: 10.1293/tox.25.135. J Toxicol Pathol. 2012. PMID: 22907980 Free PMC article.
-
Membrane blebbing as an assessment of functional rescue of dysferlin-deficient human myotubes via nonsense suppression.J Appl Physiol (1985). 2010 Sep;109(3):901-5. doi: 10.1152/japplphysiol.01366.2009. Epub 2010 Jun 17. J Appl Physiol (1985). 2010. PMID: 20558759 Free PMC article.
-
Dysferlin expression after normal myoblast transplantation in SCID and in SJL mice.Neuromuscul Disord. 2002 Feb;12(2):167-73. doi: 10.1016/s0960-8966(01)00254-1. Neuromuscul Disord. 2002. PMID: 11738359
-
Portrait of Dysferlinopathy: Diagnosis and Development of Therapy.J Clin Med. 2023 Sep 16;12(18):6011. doi: 10.3390/jcm12186011. J Clin Med. 2023. PMID: 37762951 Free PMC article. Review.
-
Progress and challenges in diagnosis of dysferlinopathy.Muscle Nerve. 2016 Nov;54(5):821-835. doi: 10.1002/mus.25367. Muscle Nerve. 2016. PMID: 27501525 Review.
Cited by
-
Identification of Novel Antisense-Mediated Exon Skipping Targets in DYSF for Therapeutic Treatment of Dysferlinopathy.Mol Ther Nucleic Acids. 2018 Dec 7;13:596-604. doi: 10.1016/j.omtn.2018.10.004. Epub 2018 Oct 11. Mol Ther Nucleic Acids. 2018. PMID: 30439648 Free PMC article.
-
Genetically confirmed limb-girdle muscular dystrophy type 2B with DYSF mutation using gene panel sequencing: A case report.Medicine (Baltimore). 2020 Jul 10;99(28):e20810. doi: 10.1097/MD.0000000000020810. Medicine (Baltimore). 2020. PMID: 32664072 Free PMC article.
-
Muscle spindle function in healthy and diseased muscle.Skelet Muscle. 2021 Jan 7;11(1):3. doi: 10.1186/s13395-020-00258-x. Skelet Muscle. 2021. PMID: 33407830 Free PMC article. Review.
-
Therapeutic Benefit of Galectin-1: Beyond Membrane Repair, a Multifaceted Approach to LGMD2B.Cells. 2021 Nov 17;10(11):3210. doi: 10.3390/cells10113210. Cells. 2021. PMID: 34831431 Free PMC article.
-
Phenotypic Drug Screening for Dysferlinopathy Using Patient-Derived Induced Pluripotent Stem Cells.Stem Cells Transl Med. 2019 Oct;8(10):1017-1029. doi: 10.1002/sctm.18-0280. Epub 2019 Jun 28. Stem Cells Transl Med. 2019. PMID: 31250983 Free PMC article.
References
-
- Liu J, Aoki M, Illa I, Wu C, Fardeau M, Angelini C, Serrano C, Urtizberea JA, Hentati F, Hamida MB, Bohlega S, Culper EJ, Amato AA, Bossie K, Oeltjen J, Bejaoui K, McKenna-Yasek D, Hosler BA, Schurr E, Arahata K, de Jong PJ, Brown RH Jr. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998 Sep;20(1):31-6. PubMed PMID:9731526. - PubMed
-
- Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, Richard I, Marchand S, Bourg N, Argov Z, Sadeh M, Mahjneh I, Marconi G, Passos-Bueno MR, Moreira Ede S, Zatz M, Beckmann JS, Bushby K. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet. 1998 Sep;20(1):37-42. PubMed PMID:9731527. - PubMed
-
- Illa I, Serrano-Munuera C, Gallardo E, Lasa A, Rojas-García R, Palmer J, Gallano P, Baiget M, Matsuda C, Brown RH. Distal anterior compartment myopathy: a dysferlin mutation causing a new muscular dystrophy phenotype. Ann Neurol. 2001 Jan;49(1):130-4. PubMed PMID:11198284. - PubMed
-
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003 May 8;423(6936):168-72. PubMed PMID:12736685. - PubMed
-
- Wang B, Yang Z, Brisson BK, Feng H, Zhang Z, Welch EM, Peltz SW, Barton ER, Brown RH Jr, Sweeney HL. Membrane blebbing as an assessment of functional rescue of dysferlin-deficient human myotubes via nonsense suppression. J Appl Physiol (1985). 2010 Sep;109(3):901-5. PubMed PMID:20558759. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

