Can formulation and drug delivery reduce attrition during drug discovery and development-review of feasibility, benefits and challenges
- PMID: 26579359
- PMCID: PMC4590717
- DOI: 10.1016/j.apsb.2013.12.003
Can formulation and drug delivery reduce attrition during drug discovery and development-review of feasibility, benefits and challenges
Abstract
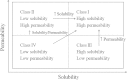
Drug discovery and development has become longer and costlier process. The fear of failure and stringent regulatory review process is driving pharmaceutical companies towards "me too" drugs and improved generics (505(b) (2)) fillings. The discontinuance of molecules at late stage clinical trials is common these years. The molecules are withdrawn at various stages of discovery and development process for reasons such as poor ADME properties, lack of efficacy and safety reasons. Hence this review focuses on possible applications of formulation and drug delivery to salvage molecules and improve the drugability. The formulation and drug delivery technologies are suitable for addressing various issues contributing to attrition are discussed in detail.
Keywords: Drug delivery technology; Drug discovery and development; Drugability; Formulation.
Figures

Similar articles
-
Methodologies for investigating drug metabolism at the early drug discovery stage: prediction of hepatic drug clearance and P450 contribution.Curr Drug Metab. 2010 Oct;11(8):678-85. doi: 10.2174/138920010794233503. Curr Drug Metab. 2010. PMID: 20973757 Review.
-
Clinical Success versus Attrition of Investigational Pharmaceuticals: A Vignette.Crit Rev Ther Drug Carrier Syst. 2017;34(6):527-549. doi: 10.1615/CritRevTherDrugCarrierSyst.2017018747. Crit Rev Ther Drug Carrier Syst. 2017. PMID: 29256836
-
Alternative strategies in drug development: clinical pharmacological aspects.Int J Clin Pharmacol Ther. 1999 Dec;37(12):575-83. Int J Clin Pharmacol Ther. 1999. PMID: 10599949 Review.
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
Improving early drug discovery through ADME modelling: an overview.Drugs R D. 2007;8(6):349-62. doi: 10.2165/00126839-200708060-00003. Drugs R D. 2007. PMID: 17963426 Review.
Cited by
-
Enhancing Whole Phage Therapy and Their Derived Antimicrobial Enzymes through Complex Formulation.Pharmaceuticals (Basel). 2018 Apr 19;11(2):34. doi: 10.3390/ph11020034. Pharmaceuticals (Basel). 2018. PMID: 29671806 Free PMC article. Review.
-
Therapeutic Potential of Thymoquinone and Its Nanoformulations in Pulmonary Injury: A Comprehensive Review.Int J Nanomedicine. 2021 Jul 27;16:5117-5131. doi: 10.2147/IJN.S314321. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34349511 Free PMC article. Review.
-
Gauging the role and impact of drug interactions and repurposing in neurodegenerative disorders.Curr Res Pharmacol Drug Discov. 2021 Apr 8;2:100022. doi: 10.1016/j.crphar.2021.100022. eCollection 2021. Curr Res Pharmacol Drug Discov. 2021. PMID: 34909657 Free PMC article. Review.
-
Local pharmaceutical research and development capacity in a developing country: a qualitative exploration of perspectives from key stakeholders in Ethiopia.J Pharm Policy Pract. 2022 Nov 24;15(1):92. doi: 10.1186/s40545-022-00491-3. J Pharm Policy Pract. 2022. PMID: 36434670 Free PMC article.
-
Design, synthesis and evaluation of 18F-labeled cationic carbonic anhydrase IX inhibitors for PET imaging.J Enzyme Inhib Med Chem. 2017 Dec;32(1):722-730. doi: 10.1080/14756366.2017.1308928. J Enzyme Inhib Med Chem. 2017. PMID: 28385087 Free PMC article.
References
-
- 〈http://www.innovation.org/drug_discovery/objects/pdf/RD_Brochure.pdf〉 [accessed on 06.06.12].
-
- Dimasi J.A. New drug development in the United States from 1963 to 1999. Clin Pharmacol Ther. 2001;69:286–296. - PubMed
-
- Dimasi J.A., Hansen R.W., Grabowski H.G. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22:151–185. - PubMed
-
- DiMasi J.A., Grabowski H.G. The cost of biopharmaceutical R & D: is biotech different? Manage Decis Econ. 2007;28:469–479.
-
- Light D.W., Warburton R. Demythologizing the high costs of pharmaceutical research. Biosocieties. 2011;6:34–50.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

