An aerosol formulation of R-salbutamol sulfate for pulmonary inhalation
- PMID: 26579368
- PMCID: PMC4590724
- DOI: 10.1016/j.apsb.2013.12.010
An aerosol formulation of R-salbutamol sulfate for pulmonary inhalation
Abstract
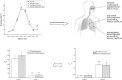
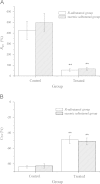
An aerosol formulation containing 7.5 mg of R-salbutamol sulfate was developed. The aerosol was nebulized with an air-jet nebulizer, and further assessed according to the new European Medicines Agency (EMA) guidelines. A breath simulator was used for studies of delivery rate and total amount of the active ingredient at volume of 3 mL. A next generation impactor (NGI) with a cooler was used for analysis of the particle size and in vitro lung deposition rate of the active ingredient at 5 °C. The anti-asthmatic efficacy of the aerosol formulation was assessed in guinea pigs with asthma evoked by intravenous injection of histamine compared with racemic salbutamol. Our results show that this aerosol formulation of R-salbutamol sulfate met all the requirements of the new EMA guidelines for nebulizer. The efficacy of a half-dose of R-salbutamol equaled that of a normal dose of racemic salbutamol.
Keywords: Asthma; Dose uniformity; Guinea pigs; NGI; Nebulizer; Particle size; R-salbutamol.
Figures

Similar articles
-
Study of pH Stability of R-Salbutamol Sulfate Aerosol Solution and Its Antiasthmatic Effects in Guinea Pigs.Biol Pharm Bull. 2017 Sep 1;40(9):1374-1380. doi: 10.1248/bpb.b17-00067. Epub 2017 Jun 24. Biol Pharm Bull. 2017. PMID: 28652557
-
Preparation of a Sustained-Release Nebulized Aerosol of R-terbutaline Hydrochloride Liposome and Evaluation of Its Anti-asthmatic Effects via Pulmonary Delivery in Guinea Pigs.AAPS PharmSciTech. 2018 Jan;19(1):232-241. doi: 10.1208/s12249-017-0816-z. Epub 2017 Jul 5. AAPS PharmSciTech. 2018. PMID: 28681333
-
Effect of Oxygen Flow on Aerosol Delivery From a Nebulizer With a Holding Chamber.Respir Care. 2019 Dec;64(12):1508-1515. doi: 10.4187/respcare.06639. Epub 2019 Aug 6. Respir Care. 2019. PMID: 31387895 Clinical Trial.
-
In-vitro/in-vivo comparison of inhaled salbutamol dose delivered by jet nebulizer, vibrating mesh nebulizer and metered dose inhaler with spacer during non-invasive ventilation.Exp Lung Res. 2017 Feb;43(1):19-28. doi: 10.1080/01902148.2017.1282993. Epub 2017 Feb 22. Exp Lung Res. 2017. PMID: 28394653
-
Fabrication, characterization and optimization of nanostructured lipid carrier formulations using Beclomethasone dipropionate for pulmonary drug delivery via medical nebulizers.Int J Pharm. 2021 Apr 1;598:120376. doi: 10.1016/j.ijpharm.2021.120376. Epub 2021 Feb 20. Int J Pharm. 2021. PMID: 33617949
Cited by
-
Effects of Non-Lethal High-Temperature Stress on Bradysia odoriphaga (Diptera: Sciaridae) Larval Development and Offspring.Insects. 2020 Mar 1;11(3):159. doi: 10.3390/insects11030159. Insects. 2020. PMID: 32121534 Free PMC article.
-
Effects of Temperature and Humidity on Laser Diffraction Measurements to Jet Nebulizer and Comparison with NGI.AAPS PharmSciTech. 2016 Apr;17(2):380-8. doi: 10.1208/s12249-015-0346-5. Epub 2015 Jul 14. AAPS PharmSciTech. 2016. PMID: 26169901 Free PMC article.
-
Development of oral dispersible tablets containing prednisolone nanoparticles for the management of pediatric asthma.Drug Des Devel Ther. 2015 Nov 20;9:5815-25. doi: 10.2147/DDDT.S86075. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26640367 Free PMC article.
-
Characterization and comparison of Re-Du-Ning aerosol particles generated by different jet nebulizers.RSC Adv. 2019 Sep 25;9(52):30292-30301. doi: 10.1039/c9ra06177k. eCollection 2019 Sep 23. RSC Adv. 2019. PMID: 35530199 Free PMC article.
References
-
- Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA., et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. 2012. Available from: 〈http:/www.cdc.gov/nchs/data/databriefs/db94.htm〉.
-
- Lin JT, Su N, Liu GL, Yin KS, Zhou X, Shen HH, etal. The impact of concomitant allergic rhinitis on asthma control: a cross-sectional nation wide survey in China. J Asthma 2013 October 4. Available from: 10.3109/02770903.2013.840789 - DOI - PubMed
-
- Chen Z.H., Wang P.L., Shen H.H. Asthma research in China: a five-year review. Respirology. 2013;18 Suppl 3:S10–S19. - PubMed
-
- Chen Y.Z. Prevention and treatment of childhood asthma in China. World J Pediatr. 2005;1:85–87.
-
- Hong J.G. Global initiative for asthma 2006. J Appl Clin Pediatr. 2007;22:1278–1280.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

