Role of mucoadhesive polymers in enhancing delivery of nimodipine microemulsion to brain via intranasal route
- PMID: 26579378
- PMCID: PMC4590727
- DOI: 10.1016/j.apsb.2014.02.002
Role of mucoadhesive polymers in enhancing delivery of nimodipine microemulsion to brain via intranasal route
Abstract
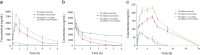
Intranasal drug administration is receiving increased attention as a delivery method for bypassing the blood-brain barrier and rapidly targeting therapeutics to the CNS. However, rapid mucociliary clearance in the nasal cavity is a major hurdle. The purpose of this study was to evaluate the effect of mucoadhesive polymers in enhancing the delivery of nimodipine microemulsion to the brain via the intranasal route. The optimized mucoadhesive microemulsion was characterized, and the in vitro drug release and in vivo nasal absorption of drug from the new formulation were evaluated in rats. The optimized formulation consisted of Capmul MCM as oil, Labrasol as surfactant, and Transcutol P as co-surfactant, with a particle size of 250 nm and zeta potential value of -15 mV. In vitro and ex vivo permeation studies showed an initial burst of drug release at 30 min and sustained release up to 6 h, attributable to the presence of free drug entrapped in the mucoadhesive layer. In vivo pharmacokinetic studies in rats showed that the use of the mucoadhesive microemulsion enhanced brain and plasma concentrations of nimodipine. These results suggest that incorporation of a mucoadhesive agent in a microemulsion intranasal delivery system can increase the retention time of the formulation and enhance brain delivery of drugs.
Keywords: Blood–brain barrier; Entrapment; Nasal mucosa; Permeation; Pharmacokinetics.
Figures







Similar articles
-
Formulation and In-vivo Pharmacokinetic Consideration of Intranasal Microemulsion and Mucoadhesive Microemulsion of Rivastigmine for Brain Targeting.Pharm Res. 2018 Jan 2;35(1):8. doi: 10.1007/s11095-017-2279-z. Pharm Res. 2018. PMID: 29294189
-
Brain-Targeted Intranasal Delivery of Zotepine Microemulsion: Pharmacokinetics and Pharmacodynamics.Pharmaceutics. 2022 Apr 30;14(5):978. doi: 10.3390/pharmaceutics14050978. Pharmaceutics. 2022. PMID: 35631564 Free PMC article.
-
Preparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain.Int J Pharm. 2004 May 4;275(1-2):85-96. doi: 10.1016/j.ijpharm.2004.01.039. Int J Pharm. 2004. PMID: 15081140
-
Convolutions in the rendition of nose to brain therapeutics from bench to bedside: Feats & fallacies.J Control Release. 2022 Jan;341:782-811. doi: 10.1016/j.jconrel.2021.12.009. Epub 2021 Dec 11. J Control Release. 2022. PMID: 34906605 Review.
-
Intranasal Drug Delivery: A Non-Invasive Approach for the Better Delivery of Neurotherapeutics.Pharm Nanotechnol. 2017;5(3):203-214. doi: 10.2174/2211738505666170515113936. Pharm Nanotechnol. 2017. PMID: 28521670 Review.
Cited by
-
Nose-to-Brain (N2B) Delivery: An Alternative Route for the Delivery of Biologics in the Management and Treatment of Central Nervous System Disorders.Pharmaceutics. 2023 Dec 31;16(1):66. doi: 10.3390/pharmaceutics16010066. Pharmaceutics. 2023. PMID: 38258077 Free PMC article. Review.
-
Systematic Approach for the Formulation and Optimization of Solid Lipid Nanoparticles of Efavirenz by High Pressure Homogenization Using Design of Experiments for Brain Targeting and Enhanced Bioavailability.Biomed Res Int. 2017;2017:5984014. doi: 10.1155/2017/5984014. Epub 2017 Jan 23. Biomed Res Int. 2017. PMID: 28243600 Free PMC article.
-
Emerging nano-derived therapy for the treatment of dementia: a comprehensive review.Drug Deliv Transl Res. 2025 Apr 23. doi: 10.1007/s13346-025-01863-3. Online ahead of print. Drug Deliv Transl Res. 2025. PMID: 40268841
-
Intranasal Drug Delivery by Nanotechnology: Advances in and Challenges for Alzheimer's Disease Management.Pharmaceutics. 2023 Dec 29;16(1):58. doi: 10.3390/pharmaceutics16010058. Pharmaceutics. 2023. PMID: 38258068 Free PMC article. Review.
-
Two kinds of ketoprofen enteric gel beads (CA and CS-SA) using biopolymer alginate.Asian J Pharm Sci. 2018 Mar;13(2):120-130. doi: 10.1016/j.ajps.2017.10.003. Epub 2017 Oct 26. Asian J Pharm Sci. 2018. PMID: 32104385 Free PMC article.
References
-
- Frey W.H., II Bypassing the blood-brain barrier to delivery therapeutic agents to the brain and spinal cord. Drug Deliv Technol. 2002;2:46–49.
-
- Thorne R.G., Pronk G.J., Padmanabhan V., Frey W.H., II Delivery of insulin like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. - PubMed
-
- Dhanda D.S., Frey W.H., II, Leopold D., Kompella U.B. Approaches for drug deposition in the human olfactory epithelium. Drug Deliv Technol. 2005;5:64–72.
-
- Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56:3–17. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

