Pharmacokinetic aspects and in vitro-in vivo correlation potential for lipid-based formulations
- PMID: 26579403
- PMCID: PMC4629105
- DOI: 10.1016/j.apsb.2014.09.001
Pharmacokinetic aspects and in vitro-in vivo correlation potential for lipid-based formulations
Abstract
Lipid-based formulations have been an attractive choice among novel drug delivery systems for enhancing the solubility and bioavailability of poorly soluble drugs due to their ability to keep the drug in solubilized state in the gastrointestinal tract. These formulations offer multiple advantages such as reduction in food effect and inter-individual variability, ease of preparation, and the possibility of manufacturing using common excipients available in the market. Despite these advantages, very few products are available in the present market, perhaps due to limited knowledge in the in vitro tests (for prediction of in vivo fate) and lack of understanding of the mechanisms behind pharmacokinetic and biopharmaceutical aspects of lipid formulations after oral administration. The current review aims to provide a detailed understanding of the in vivo processing steps involved after oral administration of lipid formulations, their pharmacokinetic aspects and in vitro in vivo correlation (IVIVC) perspectives. Various pharmacokinetic and biopharmaceutical aspects such as formulation dispersion and lipid digestion, bioavailability enhancement mechanisms, impact of excipients on efflux transporters, and lymphatic transport are discussed with examples. In addition, various IVIVC approaches towards predicting in vivo data from in vitro dispersion/precipitation, in vitro lipolysis and ex vivo permeation studies are also discussed in detail with help of case studies.
Keywords: ADME, absorption/distribution/metabolism/elimination; AUC, area under the curve; BCS, biopharmaceutics classification system; BDDCS, biopharmaceutics drug disposition classification system; CACO, human epithelial colorectal adenocarcinoma cells; CMC, critical micellar concentration; CYP, cytochrome; Cmax, maximum plasma concentration; DDS, drug delivery systems; Efflux transporters; FaSSGF, fasted-state simulated gastric fluid; FaSSIF, fasted-state simulated intestinal fluid; FeSSIF, fed-state simulated intestinal fluid; Food effect; GIT, gastrointestinal tract; IVIVC; IVIVC, in vitro in vivo correlation; LCT, long chain triglyceride; LFCS, lipid formulation classification system; Lipolysis; Lymphatic delivery; MCT, medium chain triglyceride; MDCK, Madin–Darby canine kidney cells; NCE, new chemical entity; P-app, apparent permeability; P-gp, permeability glycoprotein; Pharmacokinetics; SCT, short chain triglyceride; SEDDS, self-emulsifying drug delivery system; SIF, simulated intestinal fluid; SMEDDS, self-microemulsifying drug delivery system; SNEDDS, self-nanoemulsifying drug delivery system; Vit E, vitamin E; log P, n-octanol/water partition coefficient.
Figures


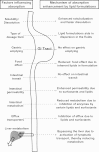
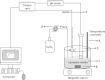

Similar articles
-
In vitro and in vivo correlation for lipid-based formulations: Current status and future perspectives.Acta Pharm Sin B. 2021 Aug;11(8):2469-2487. doi: 10.1016/j.apsb.2021.03.025. Epub 2021 Mar 21. Acta Pharm Sin B. 2021. PMID: 34522595 Free PMC article. Review.
-
Biopharmaceutical classification of poorly soluble drugs with respect to "enabling formulations".Eur J Pharm Sci. 2013 Sep 27;50(1):8-16. doi: 10.1016/j.ejps.2013.04.002. Epub 2013 Apr 11. Eur J Pharm Sci. 2013. PMID: 23583787 Review.
-
Understanding peroral absorption: regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles.Acta Pharm Sin B. 2017 May;7(3):260-280. doi: 10.1016/j.apsb.2016.09.005. Epub 2016 Nov 2. Acta Pharm Sin B. 2017. PMID: 28540164 Free PMC article. Review.
-
Analysis of the enhanced oral bioavailability of fenofibrate lipid formulations in fasted humans using an in vitro-in silico-in vivo approach.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt B):1274-84. doi: 10.1016/j.ejpb.2013.03.001. Epub 2013 Mar 14. Eur J Pharm Biopharm. 2013. PMID: 23500116
-
In vitro Methods for In vitro-In vivo Correlation (IVIVC) for Poorly Water Soluble Drugs: Lipid Based Formulation Perspective.Curr Drug Deliv. 2018;15(7):918-929. doi: 10.2174/1567201815666180116090910. Curr Drug Deliv. 2018. PMID: 29336263 Review.
Cited by
-
Bioavailability by design - Vitamin D3 liposomal delivery vehicles.Nanomedicine. 2022 Jul;43:102552. doi: 10.1016/j.nano.2022.102552. Epub 2022 Mar 26. Nanomedicine. 2022. PMID: 35346834 Free PMC article.
-
Recent Advances in Oral Nano-Antibiotics for Bacterial Infection Therapy.Int J Nanomedicine. 2020 Dec 1;15:9587-9610. doi: 10.2147/IJN.S279652. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33293809 Free PMC article. Review.
-
Lipolysis-Permeation Setup for Simultaneous Study of Digestion and Absorption in Vitro.Mol Pharm. 2019 Mar 4;16(3):921-930. doi: 10.1021/acs.molpharmaceut.8b00811. Epub 2019 Jan 29. Mol Pharm. 2019. PMID: 30628771 Free PMC article.
-
Exploring the utility of the Chasing Principle: influence of drug-free SNEDDS composition on solubilization of carvedilol, cinnarizine and R3040 in aqueous suspension.Acta Pharm Sin B. 2019 Jan;9(1):194-201. doi: 10.1016/j.apsb.2018.07.004. Epub 2018 Jul 7. Acta Pharm Sin B. 2019. PMID: 30766791 Free PMC article.
-
Development and Characterization of Innovative Nifurtimox Formulations as Therapeutic Alternative for Chagas Disease.Trop Med Infect Dis. 2025 Feb 7;10(2):50. doi: 10.3390/tropicalmed10020050. Trop Med Infect Dis. 2025. PMID: 39998054 Free PMC article.
References
-
- Fahr A., Liu X.L. Drug delivery strategies for poorly water-soluble drugs. Expert Opin Drug Deliv. 2007;4:403–416. - PubMed
-
- Lakshman J.P. Formulation, bioavailability, and manufacturing process enhancement: novel applications of melt extrusion in enabling product development. In: Michael A. Repka, Nigel Langley, James DiNunzio., editors. Melt extrusion, materials, technology and drug product design. Springer; New York: 2013. pp. 329–362.
-
- Pouton C.W. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29:278–287. - PubMed
-
- Pouton C.W. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur J Pharm Sci. 2000;11 Suppl 2:S93–S98. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

