Discovery of direct inhibitors of Keap1-Nrf2 protein-protein interaction as potential therapeutic and preventive agents
- PMID: 26579458
- PMCID: PMC4629420
- DOI: 10.1016/j.apsb.2015.05.008
Discovery of direct inhibitors of Keap1-Nrf2 protein-protein interaction as potential therapeutic and preventive agents
Abstract
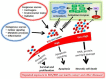
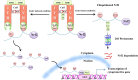
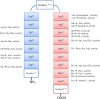
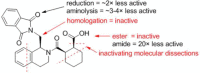
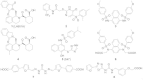
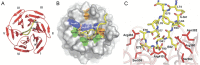
The Keap1-Nrf2-ARE pathway is an important antioxidant defense mechanism that protects cells from oxidative stress and the Keap1-Nrf2 protein-protein interaction (PPI) has become an important drug target to upregulate the expression of ARE-controlled cytoprotective oxidative stress response enzymes in the development of therapeutic and preventive agents for a number of diseases and conditions. However, most known Nrf2 activators/ARE inducers are indirect inhibitors of Keap1-Nrf2 PPI and they are electrophilic species that act by modifying the sulfhydryl groups of Keap1׳s cysteine residues. The electrophilicity of these indirect inhibitors may cause "off-target" side effects by reacting with cysteine residues of other important cellular proteins. Efforts have recently been focused on the development of direct inhibitors of Keap1-Nrf2 PPI. This article reviews these recent research efforts including the development of high throughput screening assays, the discovery of peptide and small molecule direct inhibitors, and the biophysical characterization of the binding of these inhibitors to the target Keap1 Kelch domain protein. These non-covalent direct inhibitors of Keap1-Nrf2 PPI could potentially be developed into effective therapeutic or preventive agents for a variety of diseases and conditions.
Keywords: 1O2, singlet oxygen; AD, Alzheimer׳s disease; ARE, antioxidant response element; BTB, broad complex, tramtrack and bric-a-brac; Bach1, BTB and CNC homology 1; CBP, cAMP response element binding (CREB) protein; CDDO-Me, bardoxolone methyl; COPD, chronic obstructive pulmonary disease; CTR, C-terminal region; CVD, cardiovascular disease; DGR, double glycine repeats; Direct inhibitors of protein–protein interaction; FITC, flurescein isothiocyanate; FP, fluorescence polarization; GCL, glutamate-cysteine ligase; GPx, glutathione peroxidase; GST, glutathione S-transferase; H2O2, hydrogen peroxide; HO-1, heme-oxygenase-1; HTS, high-throughput screening; High throughput screening assays; IBS, inflammatory bowel disease; IVR, intervening region; Keap1; Keap1, Kelch-like ECH-associated protein 1; MD, molecular dynamics; NMR, .; NO, nitric oxide; NQO1, NAD(P)H quinone oxidoreductase I; NTR, N-terminal region; Nrf2; Nrf2, nuclear factor erythroid 2–related factor 2; Oxidative stress; PD, Parkinson׳s disease; PPI, protein–protein interaction; RNS, reactive nitrogen species; ROS, reactive oxygen species; SOD, superoxide dismutase; SPR, surface plasmon resonance; STZ, streptozotocin; Structure–activity relationships; THIQ, tetrahydroisoquinoline; TRX, thioredoxin; X-ray crystallography; [Formula: see text], peroxynitrate; [Formula: see text], superoxide, OH·, hydroxyl radical; vitamin C, ascorbate; vitamin E, tocopherols.
Figures




References
-
- De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–185. - PubMed
-
- Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res. 2008;52 Suppl 1:S128–S138. - PubMed
-
- Lakowicz JR. 3rd ed. Springer Science and Business Media; New York: 2006. Principles of fluorescence spectroscopy.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

