Receptor tyrosine kinases: Characterisation, mechanism of action and therapeutic interests for bone cancers
- PMID: 26579483
- PMCID: PMC4620971
- DOI: 10.1016/j.jbo.2015.01.001
Receptor tyrosine kinases: Characterisation, mechanism of action and therapeutic interests for bone cancers
Abstract
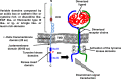
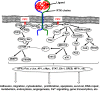
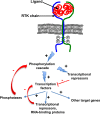
Bone cancers are characterised by the development of tumour cells in bone sites, associated with a dysregulation of their environment. In the last two decades, numerous therapeutic strategies have been developed to target the cancer cells or tumour niche. As the crosstalk between these two entities is tightly controlled by the release of polypeptide mediators activating signalling pathways through several receptor tyrosine kinases (RTKs), RTK inhibitors have been designed. These inhibitors have shown exciting clinical impacts, such as imatinib mesylate, which has become a reference treatment for chronic myeloid leukaemia and gastrointestinal tumours. The present review gives an overview of the main molecular and functional characteristics of RTKs, and focuses on the clinical applications that are envisaged and already assessed for the treatment of bone sarcomas and bone metastases.
Keywords: Bone metastasis; Bone sarcoma; Growth factor; Inhibitor; Receptor tyrosine kinase; Therapy.
Figures
Similar articles
-
Imatinib mesylate: in the treatment of gastrointestinal stromal tumours.Drugs. 2003;63(5):513-22; discussion 523-4. doi: 10.2165/00003495-200363050-00005. Drugs. 2003. PMID: 12600228 Review.
-
Evaluating the landscape of gene cooperativity with receptor tyrosine kinases in liver tumorigenesis using transposon-mediated mutagenesis.J Hepatol. 2019 Mar;70(3):470-482. doi: 10.1016/j.jhep.2018.11.027. Epub 2018 Dec 6. J Hepatol. 2019. PMID: 30529386
-
Gastrointestinal stromal tumours and their response to treatment with the tyrosine kinase inhibitor imatinib.Virchows Arch. 2004 Feb;444(2):108-18. doi: 10.1007/s00428-003-0945-5. Epub 2004 Jan 20. Virchows Arch. 2004. PMID: 14735360 Review.
-
Receptor tyrosine kinase signalling as a target for cancer intervention strategies.Endocr Relat Cancer. 2001 Sep;8(3):161-73. doi: 10.1677/erc.0.0080161. Endocr Relat Cancer. 2001. PMID: 11566607 Review.
-
Splice Variants of the RTK Family: Their Role in Tumour Progression and Response to Targeted Therapy.Int J Mol Sci. 2017 Feb 11;18(2):383. doi: 10.3390/ijms18020383. Int J Mol Sci. 2017. PMID: 28208660 Free PMC article. Review.
Cited by
-
Interstitial Deletions Generating Fusion Genes.Cancer Genomics Proteomics. 2021 May-Jun;18(3):167-196. doi: 10.21873/cgp.20251. Cancer Genomics Proteomics. 2021. PMID: 33893073 Free PMC article. Review.
-
Multi-Platform Classification of IDH-Wild-Type Glioblastoma Based on ERK/MAPK Pathway: Diagnostic, Prognostic and Therapeutic Implications.Cancers (Basel). 2021 Sep 9;13(18):4532. doi: 10.3390/cancers13184532. Cancers (Basel). 2021. PMID: 34572759 Free PMC article.
-
Design and green synthesis of novel quinolinone derivatives of potential anti-breast cancer activity against MCF-7 cell line targeting multi-receptor tyrosine kinases.J Enzyme Inhib Med Chem. 2021 Dec;36(1):1454-1471. doi: 10.1080/14756366.2021.1944126. J Enzyme Inhib Med Chem. 2021. PMID: 34210212 Free PMC article.
-
Targeting Receptor Tyrosine Kinases as a Novel Strategy for the Treatment of Triple-Negative Breast Cancer.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241234780. doi: 10.1177/15330338241234780. Technol Cancer Res Treat. 2024. PMID: 38389413 Free PMC article. Review.
-
SHP2 ablation mitigates osteoarthritic cartilage degeneration by promoting chondrocyte anabolism through SOX9.FASEB J. 2024 Sep 15;38(17):e70013. doi: 10.1096/fj.202400642R. FASEB J. 2024. PMID: 39225365
References
-
- Bhattacharya M., Babwah A.V., Ferguson S.S.G. Small GTP-binding protein-coupled receptors. Biochem Soc Trans. 2004;32:1040–1044. - PubMed
-
- Langer J.A., Cutrone E.C., Kotenko S. The class II cytokine receptor (CRF2) family: overview and patterns of receptor–ligand interactions. Cytokine Growth Factor Rev. 2004;15:33–48. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

