Unconventional Functions of Mitotic Kinases in Kidney Tumorigenesis
- PMID: 26579493
- PMCID: PMC4621426
- DOI: 10.3389/fonc.2015.00241
Unconventional Functions of Mitotic Kinases in Kidney Tumorigenesis
Abstract
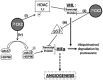
Human tumors exhibit a variety of genetic alterations, including point mutations, translocations, gene amplifications and deletions, as well as aneuploid chromosome numbers. For carcinomas, aneuploidy is associated with poor patient outcome for a large variety of tumor types, including breast, colon, and renal cell carcinoma. The Renal cell carcinoma (RCC) is a heterogeneous carcinoma consisting of different histologic types. The clear renal cell carcinoma (ccRCC) is the most common subtype and represents 85% of the RCC. Central to the biology of the ccRCC is the loss of function of the Von Hippel-Lindau gene, but is also associated with genetic instability that could be caused by abrogation of the cell cycle mitotic spindle checkpoint and may involve the Aurora kinases, which regulate centrosome maturation. Aneuploidy can also result from the loss of cell-cell adhesion and apical-basal cell polarity that also may be regulated by the mitotic kinases (polo-like kinase 1, casein kinase 2, doublecortin-like kinase 1, and Aurora kinases). In this review, we describe the "non-mitotic" unconventional functions of these kinases in renal tumorigenesis.
Keywords: Aurora-A kinase; kidney; mitotic kinases; non-mitotic functions; tumorigenesis.
Figures
Similar articles
-
Mitotic kinases: the key to duplication, segregation, and cytokinesis errors, chromosomal instability, and oncogenesis.Pharmacol Ther. 2006 Sep;111(3):974-84. doi: 10.1016/j.pharmthera.2006.02.006. Epub 2006 Apr 17. Pharmacol Ther. 2006. PMID: 16603252 Review.
-
The role of mitotic kinases in coupling the centrosome cycle with the assembly of the mitotic spindle.J Cell Sci. 2014 Oct 1;127(Pt 19):4111-22. doi: 10.1242/jcs.151753. Epub 2014 Aug 15. J Cell Sci. 2014. PMID: 25128564 Review.
-
Functional Significance of Aurora Kinases-p53 Protein Family Interactions in Cancer.Front Oncol. 2016 Nov 25;6:247. doi: 10.3389/fonc.2016.00247. eCollection 2016. Front Oncol. 2016. PMID: 27933271 Free PMC article. Review.
-
[Biologic mechanisms of mitotic abnormalities and chromosome number changes in malignant tumors].Magy Onkol. 2015 Dec;59(4):365-71. Epub 2015 Oct 25. Magy Onkol. 2015. PMID: 26665199 Hungarian.
-
Tumor suppressor VHL functions in the control of mitotic fidelity.Cancer Res. 2014 May 1;74(9):2422-31. doi: 10.1158/0008-5472.CAN-13-2040. Epub 2013 Dec 20. Cancer Res. 2014. PMID: 24362914
Cited by
-
TF-RBP-AS Triplet Analysis Reveals the Mechanisms of Aberrant Alternative Splicing Events in Kidney Cancer: Implications for Their Possible Clinical Use as Prognostic and Therapeutic Biomarkers.Int J Mol Sci. 2021 Aug 16;22(16):8789. doi: 10.3390/ijms22168789. Int J Mol Sci. 2021. PMID: 34445498 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

