Disease-Free Survival at 2 and 3 Years is a Significant Early Surrogate Marker Predicting the 5-Year Overall Survival in Patients Treated with Radical Cystectomy for Urothelial Carcinoma of the Bladder: External Evaluation and Validation in a Cohort of Korean Patients
- PMID: 26579498
- PMCID: PMC4625059
- DOI: 10.3389/fonc.2015.00246
Disease-Free Survival at 2 and 3 Years is a Significant Early Surrogate Marker Predicting the 5-Year Overall Survival in Patients Treated with Radical Cystectomy for Urothelial Carcinoma of the Bladder: External Evaluation and Validation in a Cohort of Korean Patients
Abstract
Purpose: We aimed to externally validate the association of 2- and 3-year disease-free survival (DFS) with 5-year overall survival (OS) in patients treated with radical cystectomy (RC) for urothelial carcinoma (UC) of the bladder.
Materials and methods: We reviewed the clinical data of 422 patients who underwent RC for UC of the bladder in our institution between 1991 and 2012. Survival curves were plotted with the Kaplan-Meier method. The Kappa statistic and Kendall tau-b test were used to assess the agreements between 2- and 3-year DFS and 5-year OS.
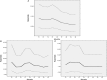
Results: In the entire study population, 2- and 3-year DFS and 5-year OS rates were 76.4, 71.5, and 67.4%, respectively. All Kappa and Kendall's tau-b test values for agreements between 2- and 3-year DFS and 5-year OS were more than 0.40, indicating moderate agreement for all patients and in each patient subgroup selected according to specific variables (all p-values <0.05). Kaplan-Meier analysis for DFS and Cox-proportional hazard models for landmark analysis at each time point indicated that most recurrences occurred within 3 years after surgery. The 5-year OS rates of patients who were recurrence-free at each time point gradually increased to more than 95% in an extended recurrence-free interval of 12-36 months.
Conclusion: Our external validation results support the existing finding that 2- and 3-year DFS can be a valid early surrogate end point to predict 5-year OS after RC in patients with UC of the bladder.
Keywords: disease-specific survival; overall survival; radical cystectomy; surrogate; urothelial carcinoma.
Figures



Similar articles
-
The Impact of Plasmacytoid Variant Histology on the Survival of Patients with Urothelial Carcinoma of Bladder after Radical Cystectomy.Eur Urol Focus. 2019 Jan;5(1):104-108. doi: 10.1016/j.euf.2017.06.013. Epub 2017 Jun 27. Eur Urol Focus. 2019. PMID: 28753857 Free PMC article.
-
Clinical Significance of Squamous Differentiation in Urothelial Carcinoma of the Bladder.Cancer Control. 2018 Jan-Dec;25(1):1073274818800269. doi: 10.1177/1073274818800269. Cancer Control. 2018. PMID: 30213195 Free PMC article.
-
External validation of disease-free survival at 2 or 3 years as a surrogate and new primary endpoint for patients undergoing radical cystectomy for urothelial carcinoma of the bladder.Eur J Surg Oncol. 2012 Jul;38(7):637-42. doi: 10.1016/j.ejso.2012.02.187. Epub 2012 Mar 27. Eur J Surg Oncol. 2012. PMID: 22459902
-
Concordance and clinical significance of uncommon variants of bladder urothelial carcinoma in transurethral resection and radical cystectomy specimens.Urology. 2014 Nov;84(5):1141-6. doi: 10.1016/j.urology.2014.06.032. Epub 2014 Sep 18. Urology. 2014. PMID: 25239253
-
The Cancer of the Bladder Risk Assessment (COBRA) score for predicting cancer-specific survival after radical cystectomy for urothelial carcinoma of the bladder: External validation in a cohort of Korean patients.Urol Oncol. 2019 Jul;37(7):470-477. doi: 10.1016/j.urolonc.2019.03.006. Epub 2019 Mar 29. Urol Oncol. 2019. PMID: 30935845
Cited by
-
Adjuvant nivolumab versus placebo following radical surgery for high-risk muscle-invasive urothelial carcinoma: a subgroup analysis of Japanese patients enrolled in the phase 3 CheckMate 274 trial.Jpn J Clin Oncol. 2023 Jan 6;53(1):16-25. doi: 10.1093/jjco/hyac155. Jpn J Clin Oncol. 2023. PMID: 36300304 Free PMC article. Clinical Trial.
-
Clinical outcomes of a cohort of patients with bulky, clinically node-positive bladder cancer undergoing radical cystectomy in the contemporary era.Can Urol Assoc J. 2021 May;15(5):E286-E289. doi: 10.5489/cuaj.6966. Can Urol Assoc J. 2021. PMID: 33119506 Free PMC article. No abstract available.
-
Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma.N Engl J Med. 2021 Jun 3;384(22):2102-2114. doi: 10.1056/NEJMoa2034442. N Engl J Med. 2021. PMID: 34077643 Free PMC article. Clinical Trial.
-
Pandemic Stressors and Adaptive Responses: A Longitudinal Analysis of the Quality of Life and Psychosocial Dynamics among Urothelial Cancer Patients.J Pers Med. 2023 Oct 28;13(11):1547. doi: 10.3390/jpm13111547. J Pers Med. 2023. PMID: 38003862 Free PMC article.
-
Adjuvant Nivolumab in High-Risk Muscle-Invasive Urothelial Carcinoma: Expanded Efficacy From CheckMate 274.J Clin Oncol. 2025 Jan;43(1):15-21. doi: 10.1200/JCO.24.00340. Epub 2024 Oct 11. J Clin Oncol. 2025. PMID: 39393026 Free PMC article. Clinical Trial.
References
-
- Calhoun A, Pohar K. Guideline-based management of muscle-invasive bladder cancer: NCCN, ICUD, and EAU. In: Konety BR, Chang SS. editors. Management of Bladder Cancer. New York, NY: Springer; (2015). p. 457–63.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

