Calcium Sensing Receptor Function Supports Osteoblast Survival and Acts as a Co-Factor in PTH Anabolic Actions in Bone
- PMID: 26579618
- PMCID: PMC4856537
- DOI: 10.1002/jcb.25447
Calcium Sensing Receptor Function Supports Osteoblast Survival and Acts as a Co-Factor in PTH Anabolic Actions in Bone
Abstract
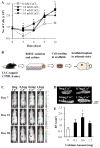
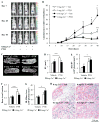
Anabolic actions of PTH in bone involve increased deposition of mineralizing matrix. Regulatory feedback of the process may be important to maintain calcium homeostasis and, in turn, calcium may inform the process. This investigation clarified the role of calcium availability and the calcium sensing receptor (CaSR) in the anabolic actions of PTH. CaSR function promoted osteoblastic cell numbers, with lower cell numbers in post-confluent cultures of primary calvarial cells from Col1-CaSR knock-out (KO) mice, and for calvarial cells from wild-type (WT) mice treated with a calcilytic. Increased apoptosis of calvarial cells with calcilytic treatment suggested CaSR is critical for protection against stage-dependent cell death. Whole and cortical, but not trabecular, bone parameters were significantly lower in Col1-CaSR KO mice versus WT littermates. Intact Col1-CaSR KO mice had lower serum P1NP levels relative to WT. PTH treatment displayed anabolic actions in WT and, to a lesser degree, KO mice, and rescued the lower P1NP levels in KO mice. Furthermore, PTH effects on whole tibiae were inhibited by osteoblast-specific CaSR ablation. Vertebral body implants (vossicles) from untreated Col1-CaSR KO and WT mice had similar bone volumes after 4 weeks of implantation in athymic mice. These findings suggest that trabecular bone formation can occur independently of the CaSR, and that the CaSR plays a collaborative role in the PTH anabolic effects on bone. J. Cell. Biochem. 117: 1556-1567, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: CALCIUM; CALCIUM SENSING RECEPTOR; OSTEOBLASTS; PARATHYROID HORMONE.
© 2015 Wiley Periodicals, Inc.
Figures



References
-
- Bennett JH, Joyner CJ, Triffitt JT, Owen ME. Adipocytic cells cultured from marrow have osteogenic potential. Journal of Cell Science. 1991;99(Pt 1):131–9. - PubMed
-
- Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. Journal of Cell Science. 1992;102(Pt 2):341–51. - PubMed
-
- Boden SD, Kaplan FS. Calcium homeostasis. Orthopedic Clinics of North America. 1990;21:31–42. - PubMed
-
- Brown EM. The calcium-sensing receptor: physiology, pathophysiology and CaR-based therapeutics. Subcellular Biochemistry. 2007;45:139–67. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

