Radiomics: Images Are More than Pictures, They Are Data
- PMID: 26579733
- PMCID: PMC4734157
- DOI: 10.1148/radiol.2015151169
Radiomics: Images Are More than Pictures, They Are Data
Abstract
In the past decade, the field of medical image analysis has grown exponentially, with an increased number of pattern recognition tools and an increase in data set sizes. These advances have facilitated the development of processes for high-throughput extraction of quantitative features that result in the conversion of images into mineable data and the subsequent analysis of these data for decision support; this practice is termed radiomics. This is in contrast to the traditional practice of treating medical images as pictures intended solely for visual interpretation. Radiomic data contain first-, second-, and higher-order statistics. These data are combined with other patient data and are mined with sophisticated bioinformatics tools to develop models that may potentially improve diagnostic, prognostic, and predictive accuracy. Because radiomics analyses are intended to be conducted with standard of care images, it is conceivable that conversion of digital images to mineable data will eventually become routine practice. This report describes the process of radiomics, its challenges, and its potential power to facilitate better clinical decision making, particularly in the care of patients with cancer.
Figures
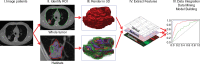
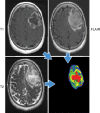

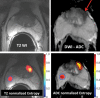
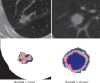
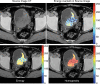
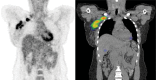
References
-
- Institute of Medicine I . Evolution of Translational Omics. Lessons learned and the path forward. Washington, DC: National Academies Press, 2012. - PubMed
-
- Giger ML. Update on the potential of computer-aided diagnosis for breast cancer. Future Oncol 2010;6(1):1–4. - PubMed
-
- Guo Z, Shu Y, Zhou H, Zhang W, Wang H. Radiogenomics helps to achieve personalized therapy by evaluating patient responses to radiation treatment. Carcinogenesis 2015;36(3):307–317. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

