Hemichordate genomes and deuterostome origins
- PMID: 26580012
- PMCID: PMC4729200
- DOI: 10.1038/nature16150
Hemichordate genomes and deuterostome origins
Abstract
Acorn worms, also known as enteropneust (literally, 'gut-breathing') hemichordates, are marine invertebrates that share features with echinoderms and chordates. Together, these three phyla comprise the deuterostomes. Here we report the draft genome sequences of two acorn worms, Saccoglossus kowalevskii and Ptychodera flava. By comparing them with diverse bilaterian genomes, we identify shared traits that were probably inherited from the last common deuterostome ancestor, and then explore evolutionary trajectories leading from this ancestor to hemichordates, echinoderms and chordates. The hemichordate genomes exhibit extensive conserved synteny with amphioxus and other bilaterians, and deeply conserved non-coding sequences that are candidates for conserved gene-regulatory elements. Notably, hemichordates possess a deuterostome-specific genomic cluster of four ordered transcription factor genes, the expression of which is associated with the development of pharyngeal 'gill' slits, the foremost morphological innovation of early deuterostomes, and is probably central to their filter-feeding lifestyle. Comparative analysis reveals numerous deuterostome-specific gene novelties, including genes found in deuterostomes and marine microbes, but not other animals. The putative functions of these genes can be linked to physiological, metabolic and developmental specializations of the filter-feeding ancestor.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
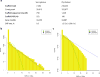

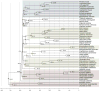

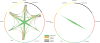

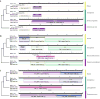
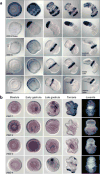


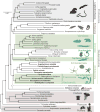
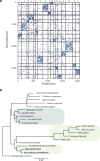

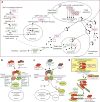
Comment in
-
Genomics: Acorn worms in a nutshell.Nature. 2015 Nov 26;527(7579):448-9. doi: 10.1038/nature16315. Epub 2015 Nov 18. Nature. 2015. PMID: 26580019 No abstract available.
References
-
- Bateson W. The later stages in the development of Balanoglossus Kowalevskii, with a suggestion as to the affinities of the Enteropneusta. 2 parts. Q J Microsc Sci. 1885;25:81–122.
-
- Bateson W. Memoirs: the ancestry of the Chordata. Q J Microsc Sci. 1886;2:535–572.
-
- Kovalevskij AO. Anatomie des Balanoglossus delle Chiaje (Mémoires de l’Académie Impériale des Sciences de. St. Pétersbourg: Imperatorskaja Akademija Nauk; 1866.
-
- Agassiz A. The history of Balanoglossus and tornaria. Memoirs of the American Academy of Arts and Sciences. 1873;9:421–436.
-
- Metschnikof V. Über die systematische Stellung von Balanoglossus. Zool Anz. 1881;4:139–157.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

