Depletion of fat-resident Treg cells prevents age-associated insulin resistance
- PMID: 26580014
- PMCID: PMC4670283
- DOI: 10.1038/nature16151
Depletion of fat-resident Treg cells prevents age-associated insulin resistance
Abstract
Age-associated insulin resistance (IR) and obesity-associated IR are two physiologically distinct forms of adult-onset diabetes. While macrophage-driven inflammation is a core driver of obesity-associated IR, the underlying mechanisms of the obesity-independent yet highly prevalent age-associated IR are largely unexplored. Here we show, using comparative adipo-immune profiling in mice, that fat-resident regulatory T cells, termed fTreg cells, accumulate in adipose tissue as a function of age, but not obesity. Supporting the existence of two distinct mechanisms underlying IR, mice deficient in fTreg cells are protected against age-associated IR, yet remain susceptible to obesity-associated IR and metabolic disease. By contrast, selective depletion of fTreg cells via anti-ST2 antibody treatment increases adipose tissue insulin sensitivity. These findings establish that distinct immune cell populations within adipose tissue underlie ageing- and obesity-associated IR, and implicate fTreg cells as adipo-immune drivers and potential therapeutic targets in the treatment of age-associated IR.
Figures

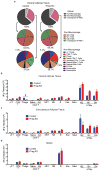
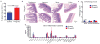


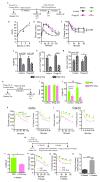
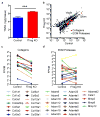
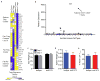
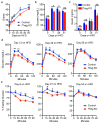

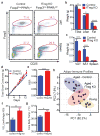
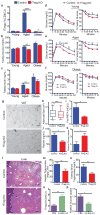

Comment in
-
Metabolism: Inflammation keeps old mice healthy.Nature. 2015 Dec 3;528(7580):44-6. doi: 10.1038/nature15648. Epub 2015 Nov 18. Nature. 2015. PMID: 26580010 No abstract available.
References
-
- Osborn O, Olefsky JM. The cellular and signaling networkslinking the immune system andmetabolism in disease. Nat Med. 2012;18:363–374. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- P01 HL088093/HL/NHLBI NIH HHS/United States
- R01 HL105278/HL/NHLBI NIH HHS/United States
- CA014195/CA/NCI NIH HHS/United States
- HL088093/HL/NHLBI NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- R56 AI099295/AI/NIAID NIH HHS/United States
- AI107027/AI/NIAID NIH HHS/United States
- R37 DK057978/DK/NIDDK NIH HHS/United States
- AI099295/AI/NIAID NIH HHS/United States
- HL105278/HL/NHLBI NIH HHS/United States
- R01 AI107027/AI/NIAID NIH HHS/United States
- DK057978/DK/NIDDK NIH HHS/United States
- R24 DK090962/DK/NIDDK NIH HHS/United States
- DK090962/DK/NIDDK NIH HHS/United States
- F30 DK096828/DK/NIDDK NIH HHS/United States
- ES010337/ES/NIEHS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- T32 GM007198/GM/NIGMS NIH HHS/United States
- R01 DK057978/DK/NIDDK NIH HHS/United States
- P30 CA014195/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

