Phosphorylation of mouse SAMHD1 regulates its restriction of human immunodeficiency virus type 1 infection, but not murine leukemia virus infection
- PMID: 26580513
- PMCID: PMC4679491
- DOI: 10.1016/j.virol.2015.10.024
Phosphorylation of mouse SAMHD1 regulates its restriction of human immunodeficiency virus type 1 infection, but not murine leukemia virus infection
Abstract
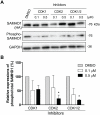
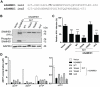
Human SAMHD1 (hSAMHD1) restricts HIV-1 infection in non-dividing cells by depleting intracellular dNTPs to limit viral reverse transcription. Phosphorylation of hSAMHD1 at threonine (T) 592 by cyclin-dependent kinase (CDK) 1 and CDK2 negatively regulates HIV-1 restriction. Mouse SAMHD1 (mSAMHD1) restricts HIV-1 infection in non-dividing cells, but whether its phosphorylation regulates retroviral restriction is unknown. Here we identified six phospho-sites of mSAMHD1, including T634 that is homologous to T592 of hSAMHD1 and phosphorylated by CDK1 and CDK2. We found that wild-type (WT) mSAMHD1 and a phospho-ablative mutant, but not a phospho-mimetic mutant, restricted HIV-1 infection in differentiated U937 cells. Murine leukemia virus (MLV) infection of dividing NIH3T3 cells was modestly restricted by mSAMHD1 WT and phospho-mutants, but not by a dNTPase-defective mutant. Our results suggest that phosphorylation of mSAMHD1 at T634 by CDK1/2 negatively regulates its HIV-1 restriction in differentiated cells, but does not affect its MLV restriction in dividing cells.
Keywords: HIV-1; Infection; MLV; Mouse and human SAMHD1; Phosphorylation; Restriction.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





References
-
- Baldauf HM, Pan X, Erikson E, Schmidt S, Daddacha W, Burggraf M, Schenkova K, Ambiel I, Wabnitz G, Gramberg T, Panitz S, Flory E, Landau NR, Sertel S, Rutsch F, Lasitschka F, Kim B, Konig R, Fackler OT, Keppler OT. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nat Med. 2012;18:1682–1687. - PMC - PubMed
-
- Behrendt R, Schumann T, Gerbaulet A, Nguyen LA, Schubert N, Alexopoulou D, Berka U, Lienenklaus S, Peschke K, Gibbert K, Wittmann S, Lindemann D, Weiss S, Dahl A, Naumann R, Dittmer U, Kim B, Mueller W, Gramberg T, Roers A. Mouse SAMHD1 has antiretroviral activity and suppresses a spontaneous cell-intrinsic antiviral response. Cell Rep. 2013;4:689–696. - PMC - PubMed
-
- Bramson HN, Corona J, Davis ST, Dickerson SH, Edelstein M, Frye SV, Gampe RT, Harris PA, Hassell A, Holmes WD, Hunter RN, Lackey KE, Lovejoy B, Luzzio MJ, Montana V, Rocque WJ, Rusnak D, Shewchuk L, Veal JM, Walker DH, Kuyper LF. Oxindole-based inhibitors of cyclin-dependent kinase 2 (CDK2): Design, synthesis, enzymatic activities, and X-ray crystallographic analysis. Journal of Medicinal Chemistry. 2001;44:4339–4358. - PubMed
-
- Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. - PubMed
-
- Cribier A, Descours B, Valadao AL, Laguette N, Benkirane M. Phosphorylation of SAMHD1 by Cyclin A2/CDK1 Regulates Its Restriction Activity toward HIV-1. Cell Rep. 2013;3:1036–1043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 AI095348/AI/NIAID NIH HHS/United States
- AI114826/AI/NIAID NIH HHS/United States
- AI095348/AI/NIAID NIH HHS/United States
- AI104483/AI/NIAID NIH HHS/United States
- R01 AI104483/AI/NIAID NIH HHS/United States
- R21 CA181997/CA/NCI NIH HHS/United States
- R01 AI120209/AI/NIAID NIH HHS/United States
- K99 AI095348/AI/NIAID NIH HHS/United States
- R01 AI049781/AI/NIAID NIH HHS/United States
- R01 GM104198/GM/NIGMS NIH HHS/United States
- AI120209/AI/NIAID NIH HHS/United States
- GM104198/GM/NIGMS NIH HHS/United States
- R56 AI049781/AI/NIAID NIH HHS/United States
- AI049781/AI/NIAID NIH HHS/United States
- CA181997/CA/NCI NIH HHS/United States
- R56 AI114826/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

