Antimicrobial Peptides from Plants
- PMID: 26580629
- PMCID: PMC4695807
- DOI: 10.3390/ph8040711
Antimicrobial Peptides from Plants
Abstract
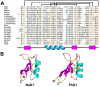
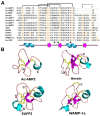
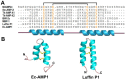
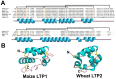
Plant antimicrobial peptides (AMPs) have evolved differently from AMPs from other life forms. They are generally rich in cysteine residues which form multiple disulfides. In turn, the disulfides cross-braced plant AMPs as cystine-rich peptides to confer them with extraordinary high chemical, thermal and proteolytic stability. The cystine-rich or commonly known as cysteine-rich peptides (CRPs) of plant AMPs are classified into families based on their sequence similarity, cysteine motifs that determine their distinctive disulfide bond patterns and tertiary structure fold. Cystine-rich plant AMP families include thionins, defensins, hevein-like peptides, knottin-type peptides (linear and cyclic), lipid transfer proteins, α-hairpinin and snakins family. In addition, there are AMPs which are rich in other amino acids. The ability of plant AMPs to organize into specific families with conserved structural folds that enable sequence variation of non-Cys residues encased in the same scaffold within a particular family to play multiple functions. Furthermore, the ability of plant AMPs to tolerate hypervariable sequences using a conserved scaffold provides diversity to recognize different targets by varying the sequence of the non-cysteine residues. These properties bode well for developing plant AMPs as potential therapeutics and for protection of crops through transgenic methods. This review provides an overview of the major families of plant AMPs, including their structures, functions, and putative mechanisms.
Keywords: cysteine-rich peptides; cystine knot; defensin; hevein; knottin; plant antimicrobial peptides; thionin.
Figures




References
-
- Ebrahim S., Usha K., Singh B. Pathogenesis related (pr) proteins in plant defense mechanism. Sci. Against Microb. Pathog. 2011;2:1043–1054.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

