The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis
- PMID: 26580649
- PMCID: PMC4663603
- DOI: 10.3390/nu7115475
The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis
Abstract
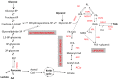
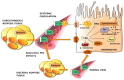
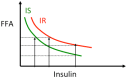
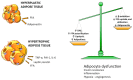
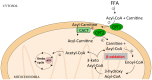
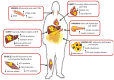
Excessive accumulation of lipids can lead to lipotoxicity, cell dysfunction and alteration in metabolic pathways, both in adipose tissue and peripheral organs, like liver, heart, pancreas and muscle. This is now a recognized risk factor for the development of metabolic disorders, such as obesity, diabetes, fatty liver disease (NAFLD), cardiovascular diseases (CVD) and hepatocellular carcinoma (HCC). The causes for lipotoxicity are not only a high fat diet but also excessive lipolysis, adipogenesis and adipose tissue insulin resistance. The aims of this review are to investigate the subtle balances that underlie lipolytic, lipogenic and oxidative pathways, to evaluate critical points and the complexities of these processes and to better understand which are the metabolic derangements resulting from their imbalance, such as type 2 diabetes and non alcoholic fatty liver disease.
Keywords: HCC; NAFLD; SCD-1; de novo lipogenesis; ectopic fat; fatty liver; glyceroneogenesis; lipolysis; lipotoxicity; saturated fat.
Figures
References
-
- Gastaldelli A. Visceral adipose tissue and ectopic fat deposition. In: Bray G.A., Bouchard C., editors. Handbook of Obesity. Volume 1. CRC Press; Boca Raton, FL, USA: 2014. pp. 237–248.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

