Adaptive Mesh Refinement and Adaptive Time Integration for Electrical Wave Propagation on the Purkinje System
- PMID: 26581455
- PMCID: PMC4637156
- DOI: 10.1155/2015/137482
Adaptive Mesh Refinement and Adaptive Time Integration for Electrical Wave Propagation on the Purkinje System
Abstract
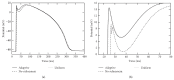
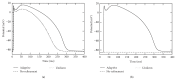
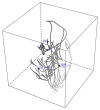
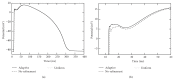
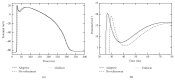
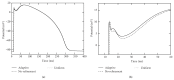
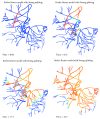
A both space and time adaptive algorithm is presented for simulating electrical wave propagation in the Purkinje system of the heart. The equations governing the distribution of electric potential over the system are solved in time with the method of lines. At each timestep, by an operator splitting technique, the space-dependent but linear diffusion part and the nonlinear but space-independent reactions part in the partial differential equations are integrated separately with implicit schemes, which have better stability and allow larger timesteps than explicit ones. The linear diffusion equation on each edge of the system is spatially discretized with the continuous piecewise linear finite element method. The adaptive algorithm can automatically recognize when and where the electrical wave starts to leave or enter the computational domain due to external current/voltage stimulation, self-excitation, or local change of membrane properties. Numerical examples demonstrating efficiency and accuracy of the adaptive algorithm are presented.
Figures


References
-
- Vigmond E. J., Clements C. Construction of a computer model to investigate sawtooth effects in the Purkinje system. IEEE Transactions on Biomedical Engineering. 2007;54(3):389–399. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

