Disruptive SCYL1 Mutations Underlie a Syndrome Characterized by Recurrent Episodes of Liver Failure, Peripheral Neuropathy, Cerebellar Atrophy, and Ataxia
- PMID: 26581903
- PMCID: PMC4678415
- DOI: 10.1016/j.ajhg.2015.10.011
Disruptive SCYL1 Mutations Underlie a Syndrome Characterized by Recurrent Episodes of Liver Failure, Peripheral Neuropathy, Cerebellar Atrophy, and Ataxia
Abstract
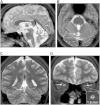
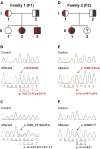
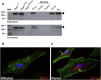
Hereditary ataxias comprise a group of genetically heterogeneous disorders characterized by clinically variable cerebellar dysfunction and accompanied by involvement of other organ systems. The molecular underpinnings for many of these diseases are widely unknown. Previously, we discovered the disruption of Scyl1 as the molecular basis of the mouse mutant mdf, which is affected by neurogenic muscular atrophy, progressive gait ataxia with tremor, cerebellar vermis atrophy, and optic-nerve thinning. Here, we report on three human individuals, from two unrelated families, who presented with recurrent episodes of acute liver failure in early infancy and are affected by cerebellar vermis atrophy, ataxia, and peripheral neuropathy. By whole-exome sequencing, compound-heterozygous mutations within SCYL1 were identified in all affected individuals. We further show that in SCYL1-deficient human fibroblasts, the Golgi apparatus is massively enlarged, which is in line with the concept that SCYL1 regulates Golgi integrity. Thus, our findings define SCYL1 mutations as the genetic cause of a human hepatocerebellar neuropathy syndrome.
Copyright © 2015 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Storey E. Genetic cerebellar ataxias. Semin. Neurol. 2014;34:280–292. - PubMed
-
- Jayadev S., Bird T.D. Hereditary ataxias: overview. Genet. Med. 2013;15:673–683. - PubMed
-
- Anheim M., Tranchant C., Koenig M. The autosomal recessive cerebellar ataxias. N. Engl. J. Med. 2012;366:636–646. - PubMed
-
- Schmidt W.M., Kraus C., Höger H., Hochmeister S., Oberndorfer F., Branka M., Bingemann S., Lassmann H., Müller M., Macedo-Souza L.I. Mutation in the Scyl1 gene encoding amino-terminal kinase-like protein causes a recessive form of spinocerebellar neurodegeneration. EMBO Rep. 2007;8:691–697. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

