Origin of Rebound Plasma HIV Includes Cells with Identical Proviruses That Are Transcriptionally Active before Stopping of Antiretroviral Therapy
- PMID: 26581989
- PMCID: PMC4719635
- DOI: 10.1128/JVI.02139-15
Origin of Rebound Plasma HIV Includes Cells with Identical Proviruses That Are Transcriptionally Active before Stopping of Antiretroviral Therapy
Abstract
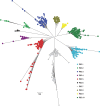
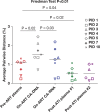
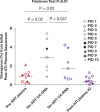
Understanding the origin of HIV variants during viral rebound may provide insight into the composition of the HIV reservoir and has implications for the design of curative interventions. HIV single-genome sequences were obtained from 10 AIDS Clinical Trials Group participants who underwent analytic antiretroviral therapy (ART) interruption (ATI). Rebounding variants were compared with those in pre-ART plasma in all 10 participants and with on-ART peripheral blood mononuclear cell (PBMC)-associated DNA and RNA (CA-RNA) in 7/10 participants. The highest viral diversities were found in the DNA and CA-RNA populations. In 3 of 7 participants, we detected multiple, identical DNA and CA-RNA sequences during suppression on ART that exactly matched plasma HIV sequences. Hypermutated DNA and CA-RNA were detected in four participants, contributing to diversities in these compartments that were higher than in the pre-ART and post-ATI plasma. Shifts in the viral rebound populations could be detected in some participants over the 2- to 3-month observation period. These findings suggest that a source of initial rebound viremia could be populations of infected cells that clonally expanded prior to and/or during ART, some of which were already expressing HIV RNA before treatment was interrupted. These clonally expanding populations of HIV-infected cells may represent an important target for strategies aimed at achieving reservoir reduction and sustained virologic remission.
Importance: Antiretroviral therapy alone cannot eradicate the HIV reservoir, and viral rebound is generally rapid after treatment interruption. It has been suggested that clonal expansion of HIV-infected cells is an important mechanism of HIV reservoir persistence, but the contribution of these clonally proliferating cells to the rebounding virus is unknown. We report a study of AIDS Clinical Trials Group participants who underwent treatment interruption and compared rebounding plasma virus with that found within cells prior to treatment interruption. We found several incidences in which plasma HIV variants exactly matched that of multiple proviral DNA copies from infected blood cells sampled before treatment interruption. In addition, we found that these cells were not dormant but were generating unspliced RNA transcripts before treatment was interrupted. Identification of the HIV reservoir and determining its mechanisms for persistence may aid in the development of strategies toward a cure for HIV. (This study was presented in part at the Conference on Retroviruses and Opportunistic Infections, Seattle, WA, February 23 to 26 2015.).
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Kearney MF, Spindler J, Shao W, Yu S, Anderson EM, O'Shea A, Rehm C, Poethke C, Kovacs N, Mellors JW, Coffin JM, Maldarelli F. 2014. Lack of detectable HIV-1 molecular evolution during suppressive antiretroviral therapy. PLoS Pathog 10:e1004010. doi:10.1371/journal.ppat.1004010. - DOI - PMC - PubMed
-
- Skiest DJ, Su Z, Havlir DV, Robertson KR, Coombs RW, Cain P, Peterson T, Krambrink A, Jahed N, McMahon D, Margolis DM. 2007. Interruption of antiretroviral treatment in HIV-infected patients with preserved immune function is associated with a low rate of clinical progression: a prospective study by AIDS Clinical Trials Group 5170. J Infect Dis 195:1426–1436. doi:10.1086/512681. - DOI - PubMed
-
- Steingrover R, Pogany K, Fernandez Garcia E, Jurriaans S, Brinkman K, Schuitemaker H, Miedema F, Lange JM, Prins JM. 2008. HIV-1 viral rebound dynamics after a single treatment interruption depends on time of initiation of highly active antiretroviral therapy. AIDS 22:1583–1588. doi:10.1097/QAD.0b013e328305bd77. - DOI - PubMed
-
- Rothenberger MK, Keele BF, Wietgrefe SW, Fletcher CV, Beilman GJ, Chipman JG, Khoruts A, Estes JD, Anderson J, Callisto SP, Schmidt TE, Thorkelson A, Reilly C, Perkey K, Reimann TG, Utay NS, Nganou Makamdop K, Stevenson M, Douek DC, Haase AT, Schacker TW. 2015. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci U S A 112:E1126–E1134. doi:10.1073/pnas.1414926112. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

