Comparative effectiveness of motivation phase intervention components for use with smokers unwilling to quit: a factorial screening experiment
- PMID: 26582140
- PMCID: PMC4681585
- DOI: 10.1111/add.13161
Comparative effectiveness of motivation phase intervention components for use with smokers unwilling to quit: a factorial screening experiment
Abstract
Aims: To screen promising intervention components designed to reduce smoking and promote abstinence in smokers initially unwilling to quit.
Design: A balanced, four-factor, randomized factorial experiment.
Setting: Eleven primary care clinics in southern Wisconsin, USA.
Participants: A total of 517 adult smokers (63.4% women, 91.1% white) recruited during primary care visits who were willing to reduce their smoking but not quit.
Interventions: Four factors contrasted intervention components designed to reduce smoking and promote abstinence: (1) nicotine patch versus none; (2) nicotine gum versus none; (3) motivational interviewing (MI) versus none; and (4) behavioral reduction counseling (BR) versus none. Participants could request cessation treatment at any point during the study.
Measurements: The primary outcome was percentage change in cigarettes smoked per day at 26 weeks post-study enrollment; the secondary outcomes were percentage change at 12 weeks and point-prevalence abstinence at 12 and 26 weeks post-study enrollment.
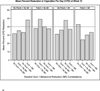
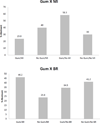
Findings: There were few main effects, but a significant four-way interaction at 26 weeks post-study enrollment (P = 0.01, β = 0.12) revealed relatively large smoking reductions by two component combinations: nicotine gum combined with BR and BR combined with MI. Further, BR improved 12-week abstinence rates (P = 0.04), and nicotine gum, when used without MI, increased 26-week abstinence after a subsequent aided quit attempt (P = 0.01).
Conclusions: Motivation-phase nicotine gum and behavioral reduction counseling are promising intervention components for smokers who are initially unwilling to quit.
Keywords: Chronic care smoking treatment; Multi-phase Optimization Strategy (MOST); Phase-Based Model; comparative effectiveness; factorial experiment; motivational interviewing; nicotine replacement therapy; primary care; smoking cessation; smoking reduction.
© 2015 Society for the Study of Addiction.
Figures



References
-
- Centers for Disease Control and Prevention. Current cigarette smoking among adults - United States, 2011. MMWR. 2012;61:889–894. - PubMed
-
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008.
-
- Bentz CJ, Bayley KB, Bonin KE, Fleming L, Hollis JF, McAfee T. The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med. 2006;30:31–37. - PubMed
-
- Etter JF, Perneger TV, Ronchi A. Distributions of smokers by stage: international comparison and association with smoking prevalence. Prev Med. 1997;26:580–585. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

