The Activation Mechanism of Glycoprotein Hormone Receptors with Implications in the Cause and Therapy of Endocrine Diseases
- PMID: 26582202
- PMCID: PMC4705372
- DOI: 10.1074/jbc.M115.701102
The Activation Mechanism of Glycoprotein Hormone Receptors with Implications in the Cause and Therapy of Endocrine Diseases
Abstract
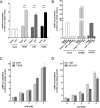
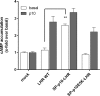
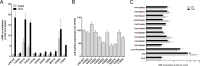
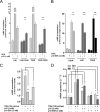
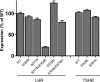
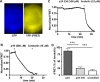
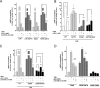
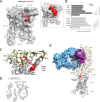
Glycoprotein hormones (GPHs) are the main regulators of the pituitary-thyroid and pituitary-gonadal axes. Selective interaction between GPHs and their cognate G protein-coupled receptors ensure specificity in GPH signaling. The mechanisms of how these hormones activate glycoprotein hormone receptors (GPHRs) or how mutations and autoantibodies can alter receptor function were unclear. Based on the hypothesis that GPHRs contain an internal agonist, we systematically screened peptide libraries derived from the ectodomain for agonistic activity on the receptors. We show that a peptide (p10) derived from a conserved sequence in the C-terminal part of the extracellular N terminus can activate all GPHRs in vitro and in GPHR-expressing tissues. Inactivating mutations in this conserved region or in p10 can inhibit activation of the thyroid-stimulating hormone receptor by autoantibodies. Our data suggest an activation mechanism where, upon extracellular ligand binding, this intramolecular agonist isomerizes and induces structural changes in the 7-transmembrane helix domain, triggering G protein activation. This mechanism can explain the pathophysiology of activating autoantibodies and several mutations causing endocrine dysfunctions such as Graves disease and hypo- and hyperthyroidism. Our findings highlight an evolutionarily conserved activation mechanism of GPHRs and will further promote the development of specific ligands useful to treat Graves disease and other dysfunctions of GPHRs.
Keywords: G protein-coupled receptor (GPCR); follicle-stimulating hormone (FSH); hormone receptor; luteinizing hormone; signal transduction; signaling; thyroid; thyroid-stimulating hormone.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Parmentier M., Libert F., Maenhaut C., Lefort A., Gérard C., Perret J., Van Sande J., Dumont J. E., and Vassart G. (1989) Molecular cloning of the thyrotropin receptor. Science 246, 1620–1622 - PubMed
-
- McFarland K. C., Sprengel R., Phillips H. S., Köhler M., Rosemblit N., Nikolics K., Segaloff D. L., and Seeburg P. H. (1989) Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science 245, 494–499 - PubMed
-
- Sprengel R., Braun T., Nikolics K., Segaloff D. L., and Seeburg P. H. (1990) The testicular receptor for follicle stimulating hormone: structure and functional expression of cloned cDNA. Mol. Endocrinol. 4, 525–530 - PubMed
-
- Sangkuhl K., Schulz A., Schultz G., and Schöneberg T. (2002) Structural requirements for mutational lutropin/choriogonadotropin receptor activation. J. Biol. Chem. 277, 47748–47755 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

