Divergent signaling via SUMO modification: potential for CFTR modulation
- PMID: 26582473
- PMCID: PMC4838058
- DOI: 10.1152/ajpcell.00124.2015
Divergent signaling via SUMO modification: potential for CFTR modulation
Abstract
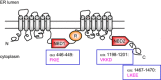
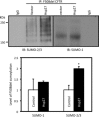
The cystic fibrosis transmembrane conductance regulator (CFTR) is generally responsible for the cAMP/PKA regulated anion conductance at the apical membranes of secretory epithelial cells. Mutations in CFTR underlie cystic fibrosis (CF), in which the most common variant, F508del, causes protein misfolding and its proteasome-mediated degradation. A new pathway that contributes to mutant CFTR degradation is mediated by the small heat shock protein, Hsp27, which cooperates with Ubc9, the E2 enzyme for SUMOylation, to selectively conjugate mutant CFTR with SUMO-2/3. This SUMO paralog can form polychains, which are recognized by the ubiquitin E3 enzyme, RNF4, leading to CFTR ubiquitylation and recognition by the proteasome. We found also that F508del CFTR could be modified by SUMO-1, a paralog that does not support SUMO polychain formation. The use of different SUMO paralogs to modify and target a single substrate for divergent purposes is not uncommon. In this short review we discuss the possibility that conjugation with SUMO-1 could protect mutant CFTR from disposal by RNF4 and similar ubiquitin ligases. We hypothesize that such a pathway could contribute to therapeutic efforts to stabilize immature mutant CFTR and thereby enhance the action of therapeutics that correct CFTR trafficking to the apical membranes.
Keywords: SIM (SUMO-interacting motif); SUMO ligase; SUMO-specific peptidase; Ubc9 (SUMO-conjugating enzyme), multi-SUMOylation.
Copyright © 2016 the American Physiological Society.
Figures
References
-
- Alkuraya FS, Saadi I, Lund JJ, Turbe-Doan A, Morton CC, Maas RL. SUMO1 haploinsufficiency leads to cleft lip and palate. Science 313: 1751, 2006. - PubMed
-
- Berta MA, Mazure N, Hattab M, Pouyssegur J, Brahimi-Horn MC. SUMOylation of hypoxia-inducible factor-1alpha reduces its transcriptional activity. Biochem Biophys Res Commun 360: 646–652, 2007. - PubMed
-
- Cappadocia L, Mascle XH, Bourdeau V, Tremblay-Belzile S, Chaker-Margot M, Lussier-Price M, Wada J, Sakaguchi K, Aubry M, Ferbeyre G, Omichinski JG. Structural and functional characterization of the phosphorylation-dependent interaction between PML and SUMO1. Structure 23: 126–138, 2015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

