Safety and Immunogenicity of Human Serum Albumin-Free MMR Vaccine in US Children Aged 12-15 Months
- PMID: 26582873
- PMCID: PMC4681379
- DOI: 10.1093/jpids/piu081
Safety and Immunogenicity of Human Serum Albumin-Free MMR Vaccine in US Children Aged 12-15 Months
Abstract
Background: M-M-R(TM)II (MMRII; Merck & Co) is currently the only measles-mumps-rubella (MMR) vaccine licensed in the United States. Another licensed vaccine would reinforce MMR supply. This study assessed the immunogenicity of a candidate vaccine (Priorix(TM), GlaxoSmithKline Vaccines [MMR-RIT]) when used as a first dose among eligible children in the United States.
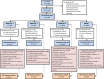
Methods: In this exploratory Phase-2, multicenter, observer-blind study, 1220 healthy subjects aged 12-15 months were randomized (3:3:3:3) and received 1 dose of 1 of 3 MMR-RIT lots with differing mumps virus titers (MMR-RIT-1 [4.8 log10]; MMR-RIT-2 [4.1 log10]; MMR-RIT-3 [3.7 log10] CCID50) or MMRII co-administered with hepatitis A vaccine (HAV), varicella vaccine (VAR) and 7-valent pneumococcal conjugate vaccine (PCV7). Immune response to measles, mumps, and rubella viruses was evaluated at Day 42 post-vaccination. Incidence of solicited injection site, general, and serious adverse events was assessed.
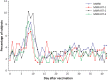
Results: Seroresponse rates for MMR vaccine viral components in MMR-RIT lots were 98.3-99.2% (measles), 89.7-90.7% (mumps), and 97.5-98.8% (rubella), and for MMRII were 99.6%, 91.1%, and 100%, respectively. Immune responses to HAV, VAR, and PCV7 were similar when co-administered with any of the 3 MMR-RIT lots or MMRII. There were no apparent differences in solicited or serious adverse events among the 4 groups.
Conclusions: Immune responses were above threshold levels for projected protection against the 3 viruses from MMR-RIT lots with differing mumps virus titers. MMR-RIT had an acceptable safety profile when co-administered with HAV, VAR, and PCV7.
Clinical trials registration: NCT00861744; etrack; 111870.
Keywords: co-administration; immunogenicity; measles; mumps; rubella.
© The Author 2014. Published by Oxford University Press on behalf of the Pediatric Infectious Diseases Society.
Figures
References
-
- Centers for Disease Control and Prevention (CDC). Summary of Notifiable Diseases, United States, 1998. MMWR Morb Mortal Wkly Rep 1999; 47:ii–92. - PubMed
-
- Centers for Disease Control and Prevention (CDC). Notes from the field: Measles outbreak—Hennepin County, Minnesota, February-March 2011. MMWR Morb Mortal Wkly Rep 2011; 60:421. - PubMed
-
- Centers for Disease Control and Prevention (CDC). Measles: United States, January-May 20, 2011. MMWR Morb Mortal Wkly Rep 2011; 60:666–8. - PubMed
-
- Dayan GH, Quinlisk MP, Parker AA, et al. Recent resurgence of mumps in the United States. N Engl J Med 2008; 358:1580–9. - PubMed
-
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention of varicella: recommendations for use of varicella vaccines in children, including a recommendation for a routine 2-dose varicella immunization schedule. Pediatrics 2007; 120:221–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

