Bioengineered vocal fold mucosa for voice restoration
- PMID: 26582902
- PMCID: PMC4669060
- DOI: 10.1126/scitranslmed.aab4014
Bioengineered vocal fold mucosa for voice restoration
Abstract
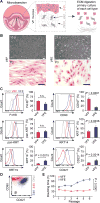
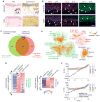
Patients with voice impairment caused by advanced vocal fold (VF) fibrosis or tissue loss have few treatment options. A transplantable, bioengineered VF mucosa would address the individual and societal costs of voice-related communication loss. Such a tissue must be biomechanically capable of aerodynamic-to-acoustic energy transfer and high-frequency vibration and physiologically capable of maintaining a barrier against the airway lumen. We isolated primary human VF fibroblasts and epithelial cells and cocultured them under organotypic conditions. The resulting engineered mucosae showed morphologic features of native tissue, proteome-level evidence of mucosal morphogenesis and emerging extracellular matrix complexity, and rudimentary barrier function in vitro. When grafted into canine larynges ex vivo, the mucosae generated vibratory behavior and acoustic output that were indistinguishable from those of native VF tissue. When grafted into humanized mice in vivo, the mucosae survived and were well tolerated by the human adaptive immune system. This tissue engineering approach has the potential to restore voice function in patients with otherwise untreatable VF mucosal disease.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures




Comment in
-
Tissue engineering. Restoring voice.Science. 2015 Nov 20;350(6263):908-9. doi: 10.1126/science.aad7695. Science. 2015. PMID: 26586745 Free PMC article. No abstract available.
References
-
- Cohen SM, Dupont WD, Courey MS. Quality-of-life impact of non-neoplastic voice disorders: a meta-analysis. Ann Otol Rhinol Laryngol. 2006;115:128–134. - PubMed
-
- Cohen SM, Kim J, Roy N, Asche C, Courey MS. The impact of laryngeal disorders on work-related dysfunction. Laryngoscope. 2012;122:1589–1594. - PubMed
-
- Cohen SM, Kim J, Roy N, Asche C, Courey MS. Direct health care costs of laryngeal diseases and disorders. Laryngoscope. 2012;122:1582–1588. - PubMed
-
- Coyle SM, Weinrich BD, Stemple JC. Shifts in relative prevalence of laryngeal pathology in a treatment-seeking population. J Voice. 2001;15:424–440. - PubMed
-
- Friedrich G, Dikkers FG, Arens C, Remacle M, Hess MM, Giovanni A, Duflo SM, Hantzakos AG, Bachy V, Gugatschka M. Vocal fold scars: current concepts and future directions. Consensus report of the phonosurgery committee of the European laryngological society. Eur Arch Otorhinolaryngol. 2013;270:2491–2507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

