Deep clinical and neuropathological phenotyping of Pick disease
- PMID: 26583316
- PMCID: PMC4755803
- DOI: 10.1002/ana.24559
Deep clinical and neuropathological phenotyping of Pick disease
Abstract
Objective: To characterize sequential patterns of regional neuropathology and clinical symptoms in a well-characterized cohort of 21 patients with autopsy-confirmed Pick disease.
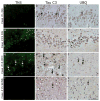
Methods: Detailed neuropathological examination using 70μm and traditional 6μm sections was performed using thioflavin-S staining and immunohistochemistry for phosphorylated tau, 3R and 4R tau isoforms, ubiquitin, and C-terminally truncated tau. Patterns of regional tau deposition were correlated with clinical data. In a subset of cases (n = 5), converging evidence was obtained using antemortem neuroimaging measures of gray and white matter integrity.
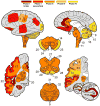
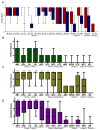
Results: Four sequential patterns of pathological tau deposition were identified starting in frontotemporal limbic/paralimbic and neocortical regions (phase I). Sequential involvement was seen in subcortical structures, including basal ganglia, locus coeruleus, and raphe nuclei (phase II), followed by primary motor cortex and precerebellar nuclei (phase III) and finally visual cortex in the most severe (phase IV) cases. Behavioral variant frontotemporal dementia was the predominant clinical phenotype (18 of 21), but all patients eventually developed a social comportment disorder. Pathological tau phases reflected the evolution of clinical symptoms and degeneration on serial antemortem neuroimaging, directly correlated with disease duration and inversely correlated with brain weight at autopsy. The majority of neuronal and glial tau inclusions were 3R tau-positive and 4R tau-negative in sporadic cases. There was a relative abundance of mature tau pathology markers in frontotemporal limbic/paralimbic regions compared to neocortical regions.
Interpretation: Pick disease tau neuropathology may originate in limbic/paralimbic cortices. The patterns of tau pathology observed here provide novel insights into the natural history and biology of tau-mediated neurodegeneration.
© 2015 American Neurological Association.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





References
-
- Pick A. Über die Beziehungen der senilen Hirnatrophie zur Aphasie. Prag Med Wochenschr. 1892;17:165–7.
-
- Kertesz A. Pick complex--historical introduction. Alzheimer disease and associated disorders. 2007 Oct-Dec;21(4):S5–7. - PubMed
-
- Yokota O, Tsuchiya K, Arai T, et al. Clinicopathological characterization of Pick’s disease versus frontotemporal lobar degeneration with ubiquitin/TDP-43-positive inclusions. Acta neuropathologica. 2009 Apr;117(4):429–44. - PubMed
-
- Piguet O, Halliday GM, Reid WG, et al. Clinical phenotypes in autopsy-confirmed Pick disease. Neurology. 2011 Jan 18;76(3):253–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS044266/NS/NINDS NIH HHS/United States
- P01 AG032953/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- R01 AG015116/AG/NIA NIH HHS/United States
- P01NS053488/NS/NINDS NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- R01 AG038490/AG/NIA NIH HHS/United States
- AG038490/AG/NIA NIH HHS/United States
- P50 NS053488/NS/NINDS NIH HHS/United States
- NS088341/NS/NINDS NIH HHS/United States
- P01AG017586/AG/NIA NIH HHS/United States
- AG010124/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- AG019724/AG/NIA NIH HHS/United States
- AG015116/AG/NIA NIH HHS/United States
- K01 AG043503/AG/NIA NIH HHS/United States
- P01AG032953/AG/NIA NIH HHS/United States
- AG023501/AG/NIA NIH HHS/United States
- NS044266/NS/NINDS NIH HHS/United States
- AG043503/AG/NIA NIH HHS/United States
- K23 NS088341/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

