A systematic literature review of individuals' perspectives on broad consent and data sharing in the United States
- PMID: 26583683
- PMCID: PMC4873460
- DOI: 10.1038/gim.2015.138
A systematic literature review of individuals' perspectives on broad consent and data sharing in the United States
Abstract
Purpose: In 2011, an Advanced Notice of Proposed Rulemaking proposed that de-identified human data and specimens be included in biobanks only if patients provide consent. The National Institutes of Health Genomic Data Sharing policy went into effect in 2015, requiring broad consent from almost all research participants.
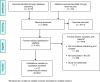
Methods: We conducted a systematic literature review of attitudes toward biobanking, broad consent, and data sharing. Bibliographic databases included MEDLINE, Web of Science, EthxWeb, and GenETHX. Study screening was conducted using DistillerSR.
Results: The final 48 studies included surveys (n = 23), focus groups (n = 8), mixed methods (n = 14), interviews (n = 1), and consent form analyses (n = 2). Study quality was characterized as good (n = 19), fair (n = 27), and poor (n = 2). Although many participants objected, broad consent was often preferred over tiered or study-specific consent, particularly when broad consent was the only option, samples were de-identified, logistics of biobanks were communicated, and privacy was addressed. Willingness for data to be shared was high, but it was lower among individuals from under-represented minorities, individuals with privacy and confidentiality concerns, and when pharmaceutical companies had access to data.
Conclusions: Additional research is needed to understand factors affecting willingness to give broad consent for biobank research and data sharing in order to address concerns to enhance acceptability.Genet Med 18 7, 663-671.
Figures
Comment in
-
Biobanks and consent with a terminal clause.Genet Med. 2016 Jul;18(7):750. doi: 10.1038/gim.2016.26. Epub 2016 Mar 31. Genet Med. 2016. PMID: 27031085 No abstract available.
-
Response to Patryn and Zagaja.Genet Med. 2016 Jul;18(7):751. doi: 10.1038/gim.2016.27. Epub 2016 Apr 14. Genet Med. 2016. PMID: 27359094 No abstract available.
References
-
- HITECH Act amendments to the HIPAA Privacy Rule. 45 C.F.R. §164.508 (c)(2015).
-
- Office of Human Research Protections. Advanced Notice of Proposed Rule Making, 76 Fed. Reg. 44512–31. 26 July 2011. http://www.gpo.gov/fdsys/pkg/FR-2011-07-26/html/2011–18792.htm. Accessed 17 April, 2015.
-
- National Institutes of Health. Genomic Data Sharing Policy, 79 Fed. Reg. 51345–54. 28 August 2014. https://www.federalregister.gov/articles/2014/08/28/2014–20385/final-nih.... Accessed 17 April, 2015.
-
- National Cancer Institute/National Action Plan on Breast Cancer. NCI/NAPBC Model Informed Consent Documents. http://oprs.usc.edu/files/2013/01/modelconsenthandouts.pdf. Accessed 17 April 2015.
-
- Tomlinson T. Respecting donors to biobank research. Hastings Cent Rep 2013;43:41–47. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 HG006385/HG/NHGRI NIH HHS/United States
- U01 HG006375/HG/NHGRI NIH HHS/United States
- U01 HG006382/HG/NHGRI NIH HHS/United States
- K01 HG008818/HG/NHGRI NIH HHS/United States
- U01 HG006389/HG/NHGRI NIH HHS/United States
- U01 HG008672/HG/NHGRI NIH HHS/United States
- U01 HG006380/HG/NHGRI NIH HHS/United States
- U01 HG008666/HG/NHGRI NIH HHS/United States
- U01 HG008673/HG/NHGRI NIH HHS/United States
- U01 HG006379/HG/NHGRI NIH HHS/United States
- U01 HG006830/HG/NHGRI NIH HHS/United States
- U01 HG008684/HG/NHGRI NIH HHS/United States
- U01 HG006828/HG/NHGRI NIH HHS/United States
- U01 HG006388/HG/NHGRI NIH HHS/United States
- U01 HG006378/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources