Simultaneous Reprogramming and Gene Correction of Patient Fibroblasts
- PMID: 26584543
- PMCID: PMC4682122
- DOI: 10.1016/j.stemcr.2015.10.009
Simultaneous Reprogramming and Gene Correction of Patient Fibroblasts
Abstract
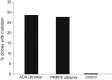
The derivation of genetically modified induced pluripotent stem (iPS) cells typically involves multiple steps, requiring lengthy cell culture periods, drug selection, and several clonal events. We report the generation of gene-targeted iPS cell lines following a single electroporation of patient-specific fibroblasts using episomal-based reprogramming vectors and the Cas9/CRISPR system. Simultaneous reprogramming and gene targeting was tested and achieved in two independent fibroblast lines with targeting efficiencies of up to 8% of the total iPS cell population. We have successfully targeted the DNMT3B and OCT4 genes with a fluorescent reporter and corrected the disease-causing mutation in both patient fibroblast lines: one derived from an adult with retinitis pigmentosa, the other from an infant with severe combined immunodeficiency. This procedure allows the generation of gene-targeted iPS cell lines with only a single clonal event in as little as 2 weeks and without the need for drug selection, thereby facilitating "seamless" single base-pair changes.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

