Teicoplanin inhibits Ebola pseudovirus infection in cell culture
- PMID: 26585243
- PMCID: PMC7113690
- DOI: 10.1016/j.antiviral.2015.11.003
Teicoplanin inhibits Ebola pseudovirus infection in cell culture
Abstract
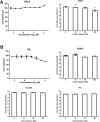
There is currently no approved antiviral therapy for treatment of Ebola virus disease. To discover readily available approved drugs that can be rapidly repurposed for treatment of Ebola virus infections, we screened 1280 FDA-approved drugs and identified glycopeptide antibiotic teicoplanin inhibiting Ebola pseudovirus infection by blocking virus entry in the low micromolar range. Teicoplanin could be evaluated further and incorporated into ongoing clinical studies.
Keywords: Antiviral drug discovery; Ebola; High-throughput screening; Pseudovirus; Teicoplanin.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures


References
-
- Altmeyer R. Virus attachment and entry offer numerous targets for antiviral therapy. Curr. Pharm. Des. 2004;10:3701–3712. - PubMed
-
- Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nature reviews. Drug Discov. 2004;3:673–683. - PubMed
-
- Baize S., Pannetier D., Oestereich L., Rieger T., Koivogui L., Magassouba N., Soropogui B., Sow M.S., Keita S., De Clerck H., Tiffany A., Dominguez G., Loua M., Traore A., Kolie M., Malano E.R., Heleze E., Bocquin A., Mely S., Raoul H., Caro V., Cadar D., Gabriel M., Pahlmann M., Tappe D., Schmidt-Chanasit J., Impouma B., Diallo A.K., Formenty P., Van Herp M., Gunther S. Emergence of Zaire Ebola virus disease in Guinea. N. Engl. J. Med. 2014;371:1418–1425. - PubMed
-
- Balzarini J., Keyaerts E., Vijgen L., Egberink H., De Clercq E., Van Ranst M., Printsevskaya S.S., Olsufyeva E.N., Solovieva S.E., Preobrazhenskaya M.N. Inhibition of feline (FIPV) and human (SARS) coronavirus by semisynthetic derivatives of glycopeptide antibiotics. Antivir. Res. 2006;72:20–33. - PMC - PubMed
-
- Balzarini J., Pannecouque C., De Clercq E., Pavlov A.Y., Printsevskaya S.S., Miroshnikova O.V., Reznikova M.I., Preobrazhenskaya M.N. Antiretroviral activity of semisynthetic derivatives of glycopeptide antibiotics. J. Med. Chem. 2003;46:2755–2764. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

