Blood eosinophils and inhaled corticosteroid/long-acting β-2 agonist efficacy in COPD
- PMID: 26585525
- PMCID: PMC4752631
- DOI: 10.1136/thoraxjnl-2015-207021
Blood eosinophils and inhaled corticosteroid/long-acting β-2 agonist efficacy in COPD
Abstract
Objective: We performed a review of studies of fluticasone propionate (FP)/salmeterol (SAL) (combination inhaled corticosteroid (ICS)/long-acting β2-agonist (LABA)) in patients with COPD, which measured baseline (pretreatment) blood eosinophil levels, to test whether blood eosinophil levels ≥2% were associated with a greater reduction in exacerbation rates with ICS therapy.
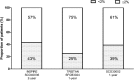
Methods: Three studies of ≥1-year duration met the inclusion criteria. Moderate and severe exacerbation rates were analysed according to baseline blood eosinophil levels (<2% vs ≥2%). At baseline, 57-75% of patients had ≥2% blood eosinophils. Changes in FEV1 and St George's Respiratory Questionnaire (SGRQ) scores were compared by eosinophil level.
Results: For patients with ≥2% eosinophils, FP/SAL was associated with significant reductions in exacerbation rates versus tiotropium (INSPIRE: n=719, rate ratio (RR)=0.75, 95% CI 0.60 to 0.92, p=0.006) and versus placebo (TRISTAN: n=1049, RR=0.63, 95% CI 0.50 to 0.79, p<0.001). No significant difference was seen in the <2% eosinophil subgroup in either study (INSPIRE: n=550, RR=1.18, 95% CI 0.92 to 1.51, p=0.186; TRISTAN: n=354, RR=0.99, 95% CI 0.67 to 1.47, p=0.957, respectively). In SCO30002 (n=373), no significant effects were observed (FP or FP/SAL vs placebo). No relationship was observed in any study between eosinophil subgroup and treatment effect on FEV1 and SGRQ.
Discussion: Baseline blood eosinophil levels may represent an informative marker for exacerbation reduction with ICS/LABA in patients with COPD and a history of moderate/severe exacerbations.
Keywords: COPD Exacerbations.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures


References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD, 2014. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-managem... (accessed 2 Sep 2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
