Epigenetic regulation of UBE3A and roles in human neurodevelopmental disorders
- PMID: 26585570
- PMCID: PMC4709177
- DOI: 10.2217/epi.15.70
Epigenetic regulation of UBE3A and roles in human neurodevelopmental disorders
Abstract
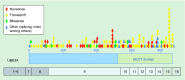
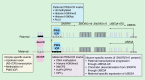
The E3 ubiquitin ligase UBE3A, also known as E6-AP, has a multitude of ascribed functions and targets relevant to human health and disease. Epigenetic regulation of the UBE3A gene by parentally imprinted noncoding transcription within human chromosome 15q11.2-q13.3 is responsible for the maternal-specific effects of 15q11.2-q13.3 deletion or duplication disorders. Here, we review the evidence for diverse and emerging roles for UBE3A in the proteasome, synapse and nucleus in regulating protein stability and transcription as well as the current mechanistic understanding of UBE3A imprinting in neurons. Angelman and Dup15q syndromes as well as experimental models of these neurodevelopmental disorders are highlighted as improving understanding of UBE3A and its complex regulation for improving therapeutic strategies.
Keywords: Angelman syndrome; Dup15q syndrome; imprinting; neurodevelopment; proteosome; ubiquitin.
Conflict of interest statement
Financial & competing interests disclosure The authors thank the NIH NINDS (R01NS076263) and the Prader–Willi Research Foundation for ongoing support of research in this area. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Figures

References
-
- Huibregtse JM, Scheffner M, Howley PM. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol. Cell. Biol. 1993;13(2):775–784. - PMC - PubMed
-
• The first paper to identify the E3 ubiqutin ligase UBE3A as a protein associated with Papillomavirus E6 protein that mediates the degredation of p53.
-
- Cooper B, Schneider S, Bohl J, Jiang Y, Beaudet A, Vande Pol S. Requirement of E6AP and the features of human papillomavirus E6 necessary to support degradation of p53. Virology. 2003;306(1):87–99. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
