Association of time to prostate-specific antigen nadir and logarithm of prostate-specific antigen velocity after progression in metastatic prostate cancer with prior primary androgen deprivation therapy
- PMID: 26585698
- PMCID: PMC5227682
- DOI: 10.4103/1008-682X.164921
Association of time to prostate-specific antigen nadir and logarithm of prostate-specific antigen velocity after progression in metastatic prostate cancer with prior primary androgen deprivation therapy
Abstract
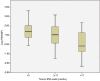
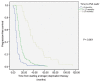
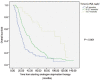
We investigated the association of time to prostate-specific antigen nadir (TTPN) and logarithm of prostate-specific antigen velocity after progression Log(PSAVAP) in metastatic prostate cancer with prior primary androgen deprivation therapy (ADT). All metastatic prostate cancer patients treated with primary ADT from 2000 to 2009 were reviewed. Patients who developed disease progression were included in the subsequent analyses. Patients were categorized into three groups according to their TTPN: TTPN of <3 months, 3-17 months, and >17 months. We compared the Log(PSAVAP) between the different TTPN groups using Mann-Whitney U-test and Kruskal-Wallis test. Further multiple linear regression analyses on Log(PSAVAP) were performed to adjust for other potential confounding factors. Among 419 patients who were treated with primary ADT, 306 patients developed disease progression with a median follow-up of 28 months. Longer TTPN was associated with lower Log(PSAVAP) (P = 0.008) within all subgroup analyses (TTPN of <3 vs 3-17 months, P= 0.020; TTPN of 3-17 vs >17 months, P= 0.009; and TTPN of <3 vs >17 months, P= 0.001). Upon multiple linear regression analyses, baseline PSA (regression coefficient 0.001, P= 0.045), PSA nadir (regression coefficient 0.002, P= 0.040), and TTPN (regression coefficient -0.030, P= 0.001) were the three factors that were significantly associated with Log(PSAVAP). In conclusion, a longer TTPN was associated with lower Log(PSAVAP) in metastatic prostate cancer patients following primary ADT. TTPN cut-offs at 3 months and 17 months appeared to have prognostic significance in predicting Log(PSAVAP). TTPN may serve as a good prognostic indicator in deciding the treatment strategy in patients with disease progression.
Figures
References
-
- Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. The Medical Research Council Prostate Cancer Working Party Investigators Group. Br J Urol. 1997;79:235–46. - PubMed
-
- Ablin RJ, Pfeiffer L, Gonder MJ, Soanes WA. Precipitating antibody in the sera of patients treated cryosurgically for carcinoma of the prostate. Exp Med Surg. 1969;27:406–10. - PubMed
-
- Nadji M, Tabei SZ, Castro A, Chu TM, Murphy GP, et al. Prostatic-specific antigen: an immunohistologic marker for prostatic neoplasms. Cancer. 1981;48:1229–32. - PubMed
-
- Artibani W. Landmarks in prostate cancer diagnosis: the biomarkers. BJU Int. 2012;110(Suppl 1):8–13. - PubMed
-
- Crawford ED, Bennett CL, Andriole GL, Garnick MB, Petrylak DP. The utility of prostate-specific antigen in the management of advanced prostate cancer. BJU Int. 2013;112:548–60. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous