Sulfatide Analysis by Mass Spectrometry for Screening of Metachromatic Leukodystrophy in Dried Blood and Urine Samples
- PMID: 26585924
- PMCID: PMC4737087
- DOI: 10.1373/clinchem.2015.245159
Sulfatide Analysis by Mass Spectrometry for Screening of Metachromatic Leukodystrophy in Dried Blood and Urine Samples
Abstract
Background: Metachromatic leukodystrophy (MLD) is an autosomal recessive disorder caused by deficiency in arylsulfatase A activity, leading to accumulation of sulfatide substrates. Diagnostic and monitoring procedures include demonstration of reduced arylsulfatase A activity in peripheral blood leukocytes or detection of sulfatides in urine. However, the development of a screening test is challenging because of instability of the enzyme in dried blood spots (DBS), the widespread occurrence of pseudodeficiency alleles, and the lack of available urine samples from newborn screening programs.
Methods: We measured individual sulfatide profiles in DBS and dried urine spots (DUS) from MLD patients with LC-MS/MS to identify markers with the discriminatory power to differentiate affected individuals from controls. We also developed a method for converting all sulfatide molecular species into a single species, allowing quantification in positive-ion mode upon derivatization.
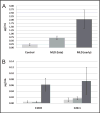
Results: In DBS from MLD patients, we found up to 23.2-fold and 5.1-fold differences in total sulfatide concentrations for early- and late-onset MLD, respectively, compared with controls and pseudodeficiencies. Corresponding DUS revealed up to 164-fold and 78-fold differences for early- and late-onset MLD patient samples compared with controls. The use of sulfatides converted to a single species simplified the analysis and increased detection sensitivity in positive-ion mode, providing a second option for sulfatide analysis.
Conclusions: This study of sulfatides in DBS and DUS suggests the feasibility of the mass spectrometry method for newborn screening of MLD and sets the stage for a larger-scale newborn screening pilot study.
© 2015 American Association for Clinical Chemistry.
Conflict of interest statement
Figures



References
-
- Fluharty AL, Meek WE, Kihara H. Pseudo arylsulfatase A deficiency: evidence for a structurally altered enzyme. Biochem Biophys Res Commun. 1983;112:191–7. - PubMed
-
- Tan MAF, Dean Caroline J, Hopwood JJ, Meikle PJ. Diagnosis of metachromatic leukodystrophy by immune quantification of arylsulphatase A protein and activity in dried blood spots. Clin Chem. 2008;54:1924–5. - PubMed
-
- Nishio H, Kodama S, Matsuo T. Analysis of fatty acids and sphingosines from urinary sulfatides in a patient with metachromatic leukodystrophy by gas chromatography-mass spectrometry. Brain Dev. 1985;7:614–21. - PubMed
-
- Natowicz MR, Prence EM, Chaturvedi P, Newburg DS. Urine sulfatides and the diagnosis of metachromatic leukodystrophy. Clin Chem. 1996;42:232–8. - PubMed
-
- Whitfield PD, Sharp PC, Johnson DW, Nelson P, Meikle PJ. Characterization of urinary sulfatides in metachromatic leukodystrophy using electrospray ionization-tandem mass spectrometry. Mol Genet Metab. 2001;73:30–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

