Flexible Sensory Representations in Auditory Cortex Driven by Behavioral Relevance
- PMID: 26586181
- PMCID: PMC4670799
- DOI: 10.1016/j.neuron.2015.10.024
Flexible Sensory Representations in Auditory Cortex Driven by Behavioral Relevance
Abstract
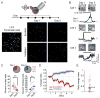
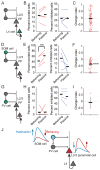
Animals require the ability to ignore sensory stimuli that have no consequence yet respond to the same stimuli when they become useful. However, the brain circuits that govern this flexibility in sensory processing are not well understood. Here we show in mouse primary auditory cortex (A1) that daily passive sound exposure causes a long-lasting reduction in representations of the experienced sound by layer 2/3 pyramidal cells. This habituation arises locally in A1 and involves an enhancement in inhibition and selective upregulation in the activity of somatostatin-expressing inhibitory neurons (SOM cells). Furthermore, when mice engage in sound-guided behavior, pyramidal cell excitatory responses to habituated sounds are enhanced, whereas SOM cell responses are diminished. Together, our results demonstrate the bidirectional modulation of A1 sensory representations and suggest that SOM cells gate cortical information flow based on the behavioral relevance of the stimulus.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





References
-
- Bakin JS, South DA, Weinberger NM. Induction of receptive field plasticity in the auditory cortex of the guinea pig during instrumental avoidance conditioning. Behavioral Neuroscience. 1996;110:905–913. - PubMed
-
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

