Ibuprofen slows migration and inhibits bowel colonization by enteric nervous system precursors in zebrafish, chick and mouse
- PMID: 26586201
- PMCID: PMC4862364
- DOI: 10.1016/j.ydbio.2015.09.023
Ibuprofen slows migration and inhibits bowel colonization by enteric nervous system precursors in zebrafish, chick and mouse
Abstract
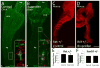
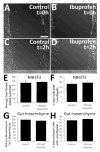
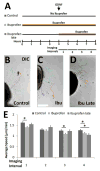
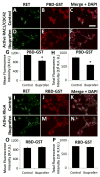
Hirschsprung Disease (HSCR) is a potentially deadly birth defect characterized by the absence of the enteric nervous system (ENS) in distal bowel. Although HSCR has clear genetic causes, no HSCR-associated mutation is 100% penetrant, suggesting gene-gene and gene-environment interactions determine HSCR occurrence. To test the hypothesis that certain medicines might alter HSCR risk we treated zebrafish with medications commonly used during early human pregnancy and discovered that ibuprofen caused HSCR-like absence of enteric neurons in distal bowel. Using fetal CF-1 mouse gut slice cultures, we found that ibuprofen treated enteric neural crest-derived cells (ENCDC) had reduced migration, fewer lamellipodia and lower levels of active RAC1/CDC42. Additionally, inhibiting ROCK, a RHOA effector and known RAC1 antagonist, reversed ibuprofen effects on migrating mouse ENCDC in culture. Ibuprofen also inhibited colonization of Ret+/- mouse bowel by ENCDC in vivo and dramatically reduced bowel colonization by chick ENCDC in culture. Interestingly, ibuprofen did not affect ENCDC migration until after at least three hours of exposure. Furthermore, mice deficient in Ptgs1 (COX 1) and Ptgs2 (COX 2) had normal bowel colonization by ENCDC and normal ENCDC migration in vitro suggesting COX-independent effects. Consistent with selective and strain specific effects on ENCDC, ibuprofen did not affect migration of gut mesenchymal cells, NIH3T3, or WT C57BL/6 ENCDC, and did not affect dorsal root ganglion cell precursor migration in zebrafish. Thus, ibuprofen inhibits ENCDC migration in vitro and bowel colonization by ENCDC in vivo in zebrafish, mouse and chick, but there are cell type and strain specific responses. These data raise concern that ibuprofen may increase Hirschsprung disease risk in some genetically susceptible children.
Keywords: Enteric nervous system development; Gene-environment interactions; Migration.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures






References
-
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. - PubMed
-
- Aluri JB, Stavchansky S. Determination of guaifenesin in human plasma by liquid chromatography in the presence of pseudoephedrine. J Pharm Biomed Anal. 1993;11:803–808. - PubMed
-
- Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, Pelet A, Arnold S, Miao X, Griseri P, Brooks AS, Antinolo G, de Pontual L, Clement-Ziza M, Munnich A, Kashuk C, West K, Wong KK, Lyonnet S, Chakravarti A, Tam PK, Ceccherini I, Hofstra RM, Fernandez R. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45:1–14. - PubMed
-
- Anderson RB, Newgreen DF, Young HM. Neural crest and the development of the enteric nervous system. Adv Exp Med Biol. 2006;589:181–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

