Characterization of circulating APOL1 protein complexes in African Americans
- PMID: 26586272
- PMCID: PMC4689339
- DOI: 10.1194/jlr.M063453
Characterization of circulating APOL1 protein complexes in African Americans
Abstract
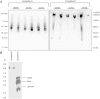
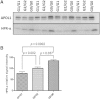
APOL1 gene renal-risk variants are associated with nephropathy and CVD in African Americans; however, little is known about the circulating APOL1 variant proteins which reportedly bind to HDL. We examined whether APOL1 G1 and G2 renal-risk variant serum concentrations or lipoprotein distributions differed from nonrisk G0 APOL1 in African Americans without nephropathy. Serum APOL1 protein concentrations were similar regardless of APOL1 genotype. In addition, serum APOL1 protein was bound to protein complexes in two nonoverlapping peaks, herein referred to as APOL1 complex A (12.2 nm diameter) and complex B (20.0 nm diameter). Neither of these protein complexes associated with HDL or LDL. Proteomic analysis revealed that complex A was composed of APOA1, haptoglobin-related protein (HPR), and complement C3, whereas complex B contained APOA1, HPR, IgM, and fibronectin. Serum HPR was less abundant on complex B in individuals with G1 and G2 renal-risk variant genotypes, relative to G0 (P = 0.0002-0.037). These circulating complexes may play roles in HDL metabolism and susceptibility to CVD.
Keywords: apolipoprotein L1; apolipoproteins; high density lipoprotein; kidney; proteomics; renal disease.
Copyright © 2016 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
-
- Tzur S., Rosset S., Shemer R., Yudkovsky G., Selig S., Tarekegn A., Bekele E., Bradman N., Wasser W. G., Behar D. M., et al. . 2010. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum. Genet. 128: 345–350. - PMC - PubMed
-
- Ma L., Shelness G. S., Snipes J. A., Murea M., Antinozzi P. A., Cheng D., Saleem M. A., Satchell S. C., Banas B., Mathieson P. W., et al. . 2015. Localization of APOL1 protein and mRNA in the human kidney: nondiseased tissue, primary cells, and immortalized cell lines. J. Am. Soc. Nephrol. 26: 339–348. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

