Photoreactive helical nanoaggregates exhibiting morphology transition on thermal reconstruction
- PMID: 26586298
- PMCID: PMC4673833
- DOI: 10.1038/ncomms9936
Photoreactive helical nanoaggregates exhibiting morphology transition on thermal reconstruction
Abstract
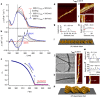
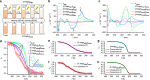
The supramolecular design of photochromic molecules has produced various smart molecular assemblies that can switch their structures and/or functions in response to light stimuli. However, most of these assemblies require large structural changes of the photochromic molecules for an efficient conversion of assembled states, which often suppresses the photoreactivity within the self-assemblies. Here we report molecular assemblies, based on a photo-cross-linkable chromophoric dyad, in which a small amount of ultraviolet-generated photochemical product can guide the entire system into different assembly processes. In apolar solution, the intact dyad self-assembles into right-handed superhelical fibrils. On ultraviolet-irradiation of these fibrils, an effective photoreaction affords a sole photo-cross-linked product. When right-handed helical fibrils, containing a minor amount of the photoproduct, are thermally reconstructed, the intact molecule and the photoproduct undergo a co-assembly process that furnishes superhelical fibrils with different molecular packing structures. This molecular design principle should afford new paradigms for smart molecular assemblies.
Figures




References
-
- Ramamurthy V. & Inoue Y. Supramolecular Photochemistry: Controlling Photochemical Processes Wiley (2011).
-
- Hoeben F. J. M., Jonkheijm P., Meijer E. W. & Schenning A. P. H. J. About supramolecular assemblies of π-conjugated systems. Chem. Rev. 105, 1491–1546 (2005). - PubMed
-
- Rosen B. M. et al.. Dendron-mediated self-assembly, disassembly, and self-organization of complex systems. Chem. Rev. 109, 6275–6540 (2009). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

