Single-cell analysis and stochastic modelling unveil large cell-to-cell variability in influenza A virus infection
- PMID: 26586423
- PMCID: PMC4673863
- DOI: 10.1038/ncomms9938
Single-cell analysis and stochastic modelling unveil large cell-to-cell variability in influenza A virus infection
Abstract
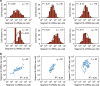
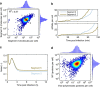
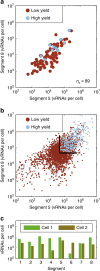
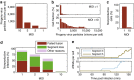
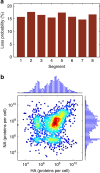
Biochemical reactions are subject to stochastic fluctuations that can give rise to cell-to-cell variability. Yet, how this variability affects viral infections, which themselves involve noisy reactions, remains largely elusive. Here we present single-cell experiments and stochastic simulations that reveal a large heterogeneity between influenza A virus (IAV)-infected cells. In particular, experimental data show that progeny virus titres range from 1 to 970 plaque-forming units and intracellular viral RNA (vRNA) levels span three orders of magnitude. Moreover, the segmentation of IAV genomes seems to increase the susceptibility of their replication to noise, since the level of different genome segments can vary substantially within a cell. In addition, simulations suggest that the abortion of virus entry and random degradation of vRNAs can result in a large fraction of non-productive cells after single-hit infection. These results challenge current beliefs that cell population measurements and deterministic simulations are an accurate representation of viral infections.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

