Chromatin Dynamics and the RNA Exosome Function in Concert to Regulate Transcriptional Homeostasis
- PMID: 26586442
- PMCID: PMC4662874
- DOI: 10.1016/j.celrep.2015.10.030
Chromatin Dynamics and the RNA Exosome Function in Concert to Regulate Transcriptional Homeostasis
Abstract
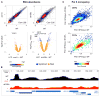
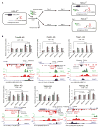
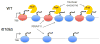
The histone variant H2A.Z is a hallmark of nucleosomes flanking promoters of protein-coding genes and is often found in nucleosomes that carry lysine 56-acetylated histone H3 (H3-K56Ac), a mark that promotes replication-independent nucleosome turnover. Here, we find that H3-K56Ac promotes RNA polymerase II occupancy at many protein-coding and noncoding loci, yet neither H3-K56Ac nor H2A.Z has a significant impact on steady-state mRNA levels in yeast. Instead, broad effects of H3-K56Ac or H2A.Z on RNA levels are revealed only in the absence of the nuclear RNA exosome. H2A.Z is also necessary for the expression of divergent, promoter-proximal noncoding RNAs (ncRNAs) in mouse embryonic stem cells. Finally, we show that H2A.Z functions with H3-K56Ac to facilitate formation of chromosome interaction domains (CIDs). Our study suggests that H2A.Z and H3-K56Ac work in concert with the RNA exosome to control mRNA and ncRNA expression, perhaps in part by regulating higher-order chromatin structures.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Albert I, Mavrich TN, Tomsho LP, Qi J, Zanton SJ, Schuster SC, Pugh BF. Translational and rotational settings of H2A.Z nucleosomes across the Saccharomyces cerevisiae genome. Nature. 2007;446:572–576. - PubMed
-
- Amberg DC, Burke DJ, Strathern JN. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, 2005 Edition. Cold Spring Harbor Laboratory Press; 2005.
-
- Arigo JT, Eyler DE, Carroll KL, Corden JL. Termination of cryptic unstable transcripts is directed by yeast RNA-binding proteins Nrd1 and Nab3. Mol Cell. 2006;23:841–851. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

