The Role of Prolactin in Bone Metastasis and Breast Cancer Cell-Mediated Osteoclast Differentiation
- PMID: 26586670
- PMCID: PMC5943829
- DOI: 10.1093/jnci/djv338
The Role of Prolactin in Bone Metastasis and Breast Cancer Cell-Mediated Osteoclast Differentiation
Abstract
Background: Metastasis to the bone is a deleterious aspect of breast cancer and is a preferred site that results in bone loss. Hormones such as prolactin (PRL) have not yet been studied for their role in modulating the secondary tumor bone microenvironment.
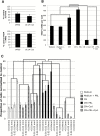
Methods: We used quantitative immunohistochemistry with 134 samples of human primary breast cancer and 17 matched primary breast cancers and bone metastases. A Cox proportional hazards regression model was fitted to evaluate the associations between high prolactin receptor (PRLR) expression and time to bone metastasis, adjusting for estrogen receptor status, lymph node status, and chemotherapy status. We assessed osteoclast differentiation, osteoclast size, and measured pit formation in dentine slices. Statistical tests were two-sided.
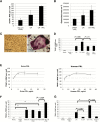
Results: High PRLR expression in the primary breast tumor was associated with a shorter time to metastasis that includes bone (PRLRAQUA Max-per 100 unit hazard ratio = 1.04, 95% confidence interval = 1.00 to 1.07, P = .03). We observed the PRLR in rare samples of bone metastases and matched primary breast cancer. PRL treatment of breast cancer cells induced osteoclast differentiation and bone lysis via secreted factors and was abrogated by a PRLR antagonist (delta1-9-G129R-hPRL). We demonstrated that sonic hedgehog is a PRL-regulated cytokine in breast cancer cells and part of the mechanism that induces osteoclast differentiation.
Conclusions: Our evidence indicates that PRL-PRLR can escalate the impact of breast cancer on bone metastasis and that the presence of the PRLR in the tumor microenvironment of breast cancer bone metastasis has the potential to modulate the microenvironment to induce lytic osteoclast formation.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
What Is Breast in the Bone?Int J Mol Sci. 2016 Oct 22;17(10):1764. doi: 10.3390/ijms17101764. Int J Mol Sci. 2016. PMID: 27782069 Free PMC article. Review.
-
A favorable role of prolactin in human breast cancer reveals novel pathway-based gene signatures indicative of tumor differentiation and favorable patient outcome.Hum Pathol. 2016 Jul;53:142-52. doi: 10.1016/j.humpath.2016.02.010. Epub 2016 Mar 14. Hum Pathol. 2016. PMID: 26980025
-
Growth hormone signaling in human T47D breast cancer cells: potential role for a growth hormone receptor-prolactin receptor complex.Mol Endocrinol. 2011 Apr;25(4):597-610. doi: 10.1210/me.2010-0255. Epub 2011 Feb 10. Mol Endocrinol. 2011. PMID: 21310852 Free PMC article.
-
Prolactin Receptor Expression is an Independent Favorable Prognostic Marker in Human Breast Cancer.Appl Immunohistochem Mol Morphol. 2016 Apr;24(4):238-45. doi: 10.1097/PAI.0000000000000178. Appl Immunohistochem Mol Morphol. 2016. PMID: 26317306
-
Prolactin receptor gene transcriptional control, regulatory modalities relevant to breast cancer resistance and invasiveness.Front Endocrinol (Lausanne). 2022 Sep 15;13:949396. doi: 10.3389/fendo.2022.949396. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36187116 Free PMC article. Review.
Cited by
-
Kadsurenone is a useful and promising treatment strategy for breast cancer bone metastases by blocking the PAF/PTAFR signaling pathway.Oncol Lett. 2018 Aug;16(2):2255-2262. doi: 10.3892/ol.2018.8935. Epub 2018 Jun 8. Oncol Lett. 2018. PMID: 30008927 Free PMC article.
-
Genome-wide Association Study Identified Chromosome 8 Locus Associated with Medication-Related Osteonecrosis of the Jaw.Clin Pharmacol Ther. 2021 Dec;110(6):1558-1569. doi: 10.1002/cpt.2397. Epub 2021 Aug 31. Clin Pharmacol Ther. 2021. PMID: 34390503 Free PMC article.
-
Prognostic Differences of Adjuvant Radiotherapy in Breast Cancer Cohorts Based on PRLR Genotypes, Expression, and Transcriptional Network Regulation.Cancers (Basel). 2025 Jul 17;17(14):2378. doi: 10.3390/cancers17142378. Cancers (Basel). 2025. PMID: 40723262 Free PMC article.
-
Current and Emerging Biomarkers Predicting Bone Metastasis Development.Front Oncol. 2020 Jun 3;10:789. doi: 10.3389/fonc.2020.00789. eCollection 2020. Front Oncol. 2020. PMID: 32582538 Free PMC article. Review.
-
Prolactin receptor signaling: A novel target for cancer treatment - Exploring anti-PRLR signaling strategies.Front Endocrinol (Lausanne). 2023 Jan 13;13:1112987. doi: 10.3389/fendo.2022.1112987. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36714582 Free PMC article. Review.
References
-
- Kingsley LA, Fournier PG, Chirgwin JM, et al. Molecular biology of bone metastasis . Mol Cancer Ther . 2007. ; 6(10) : 2609 – 2617 . - PubMed
-
- Clement-Lacroix P, Ormandy C, Lepescheux L, et al. Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice . Endocrinology . 1999. ; 140(1) : 96 – 105 . - PubMed
-
- Coss D, Yang L, Kuo CB, et al. Effects of prolactin on osteoblast alkaline phosphatase and bone formation in the developing rat . Am J Physiol Endocrinol Metab . 2000. ; 279(6) : E1216 - E1225 . - PubMed
-
- Kelly PA, Binart N, Freemark M, et al. Prolactin receptor signal transduction pathways and actions determined in prolactin receptor knockout mice . Biochem Soc Trans . 2001. ; 29 ( Pt 2 ): 48 – 52 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

