Cascading network failure across the Alzheimer's disease spectrum
- PMID: 26586695
- PMCID: PMC4805086
- DOI: 10.1093/brain/awv338
Cascading network failure across the Alzheimer's disease spectrum
Abstract
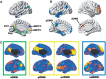
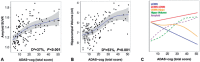
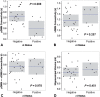
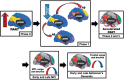
Complex biological systems are organized across various spatiotemporal scales with particular scientific disciplines dedicated to the study of each scale (e.g. genetics, molecular biology and cognitive neuroscience). When considering disease pathophysiology, one must contemplate the scale at which the disease process is being observed and how these processes impact other levels of organization. Historically Alzheimer's disease has been viewed as a disease of abnormally aggregated proteins by pathologists and molecular biologists and a disease of clinical symptoms by neurologists and psychologists. Bridging the divide between these scales has been elusive, but the study of brain networks appears to be a pivotal inroad to accomplish this task. In this study, we were guided by an emerging systems-based conceptualization of Alzheimer's disease and investigated changes in brain networks across the disease spectrum. The default mode network has distinct subsystems with unique functional-anatomic connectivity, cognitive associations, and responses to Alzheimer's pathophysiology. These distinctions provide a window into the systems-level pathophysiology of Alzheimer's disease. Using clinical phenotyping, metadata, and multimodal neuroimaging data from the Alzheimer's Disease Neuroimaging Initiative, we characterized the pattern of default mode network subsystem connectivity changes across the entire disease spectrum (n = 128). The two main findings of this paper are (i) the posterior default mode network fails before measurable amyloid plaques and appears to initiate a connectivity cascade that continues throughout the disease spectrum; and (ii) high connectivity between the posterior default mode network and hubs of high connectivity (many located in the frontal lobe) is associated with amyloid accumulation. These findings support a system model best characterized by a cascading network failure--analogous to cascading failures seen in power grids triggered by local overloads proliferating to downstream nodes eventually leading to widespread power outages, or systems failures. The failure begins in the posterior default mode network, which then shifts processing burden to other systems containing prominent connectivity hubs. This model predicts a connectivity 'overload' that precedes structural and functional declines and recasts the interpretation of high connectivity from that of a positive compensatory phenomenon to that of a load-shifting process transiently serving a compensatory role. It is unknown whether this systems-level pathophysiology is the inciting event driving downstream molecular events related to synaptic activity embedded in these systems. Possible interpretations include that the molecular-level events drive the network failure, a pathological interaction between the network-level and the molecular-level, or other upstream factors are driving both.
Keywords: Alzheimer’s disease; cascading failure; complex systems; default mode network; pathophysiology.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



References
-
- Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol Aging 2012; 33: 1564–78. - PubMed
-
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38: 95–113. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239–59. - PubMed

