Identification of Selective Lead Compounds for Treatment of High-Ploidy Breast Cancer
- PMID: 26586723
- PMCID: PMC4707107
- DOI: 10.1158/1535-7163.MCT-15-0527
Identification of Selective Lead Compounds for Treatment of High-Ploidy Breast Cancer
Abstract
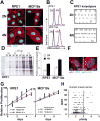
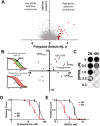
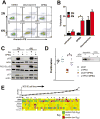
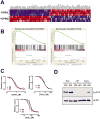
Increased ploidy is common in tumors but treatments for tumors with excess chromosome sets are not available. Here, we characterize high-ploidy breast cancers and identify potential anticancer compounds selective for the high-ploidy state. Among 354 human breast cancers, 10% have mean chromosome copy number exceeding 3, and this is most common in triple-negative and HER2-positive types. Women with high-ploidy breast cancers have higher risk of recurrence and death in two patient cohorts, demonstrating that it represents an important group for improved treatment. Because high-ploidy cancers are aneuploid, rather than triploid or tetraploid, we devised a two-step screen to identify selective compounds. The screen was designed to assure both external validity on diverse karyotypic backgrounds and specificity for high-ploidy cell types. This screen identified novel therapies specific to high-ploidy cells. First, we discovered 8-azaguanine, an antimetabolite that is activated by hypoxanthine phosphoribosyltransferase 1 (HPRT1), suggesting an elevated gene-dosage of HPRT1 in high-ploidy tumors can control sensitivity to this drug. Second, we discovered a novel compound, 2,3-diphenylbenzo[g]quinoxaline-5,10-dione (DPBQ). DPBQ activates p53 and triggers apoptosis in a polyploid-specific manner, but does not inhibit topoisomerase or bind DNA. Mechanistic analysis demonstrates that DPBQ elicits a hypoxia gene signature and its effect is replicated, in part, by enhancing oxidative stress. Structure-function analysis defines the core benzo[g]quinoxaline-5,10 dione as being necessary for the polyploid-specific effects of DPBQ. We conclude that polyploid breast cancers represent a high-risk subgroup and that DPBQ provides a functional core to develop polyploid-selective therapy. Mol Cancer Ther; 15(1); 48-59. ©2015 AACR.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

