Identification of Arabidopsis GPAT9 (At5g60620) as an Essential Gene Involved in Triacylglycerol Biosynthesis
- PMID: 26586834
- PMCID: PMC4704598
- DOI: 10.1104/pp.15.01563
Identification of Arabidopsis GPAT9 (At5g60620) as an Essential Gene Involved in Triacylglycerol Biosynthesis
Abstract
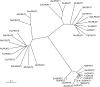
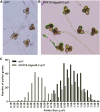
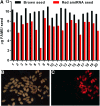
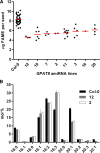
The first step in the biosynthesis of nearly all plant membrane phospholipids and storage triacylglycerols is catalyzed by a glycerol-3-phosphate acyltransferase (GPAT). The requirement for an endoplasmic reticulum (ER)-localized GPAT for both of these critical metabolic pathways was recognized more than 60 years ago. However, identification of the gene(s) encoding this GPAT activity has remained elusive. Here, we present the results of a series of in vivo, in vitro, and in silico experiments in Arabidopsis (Arabidopsis thaliana) designed to assign this essential function to AtGPAT9. This gene has been highly conserved throughout evolution and is largely present as a single copy in most plants, features consistent with essential housekeeping functions. A knockout mutant of AtGPAT9 demonstrates both male and female gametophytic lethality phenotypes, consistent with the role in essential membrane lipid synthesis. Significant expression of developing seed AtGPAT9 is required for wild-type levels of triacylglycerol accumulation, and the transcript level is directly correlated to the level of microsomal GPAT enzymatic activity in seeds. Finally, the AtGPAT9 protein interacts with other enzymes involved in ER glycerolipid biosynthesis, suggesting the possibility of ER-localized lipid biosynthetic complexes. Together, these results suggest that GPAT9 is the ER-localized GPAT enzyme responsible for plant membrane lipid and oil biosynthesis.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures



References
-
- Allen DK, Bates PD, Tjellström H (2015) Tracking the metabolic pulse of plant lipid production with isotopic labeling and flux analyses: past, present and future. Prog Lipid Res 58: 97–120 - PubMed
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Barron EJ, Stumpf PK (1962) Fat metabolism in higher plants. XIX. The biosynthesis of triglycerides by avocado-mesocarp enzymes. Biochim Biophys Acta 60: 329–337 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

