The Relay/Converter Interface Influences Hydrolysis of ATP by Skeletal Muscle Myosin II
- PMID: 26586917
- PMCID: PMC4722456
- DOI: 10.1074/jbc.M115.688002
The Relay/Converter Interface Influences Hydrolysis of ATP by Skeletal Muscle Myosin II
Abstract
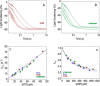
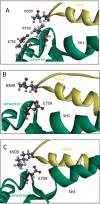
The interface between relay and converter domain of muscle myosin is critical for optimal myosin performance. Using Drosophila melanogaster indirect flight muscle S1, we performed a kinetic analysis of the effect of mutations in the converter and relay domain. Introduction of a mutation (R759E) in the converter domain inhibits the steady-state ATPase of myosin S1, whereas an additional mutation in the relay domain (N509K) is able to restore the ATPase toward wild-type values. The R759E S1 construct showed little effect on most steps of the actomyosin ATPase cycle. The exception was a 25-30% reduction in the rate constant of the hydrolysis step, the step coupled to the cross-bridge recovery stroke that involves a change in conformation at the relay/converter domain interface. Significantly, the double mutant restored the hydrolysis step to values similar to the wild-type myosin. Modeling the relay/converter interface suggests a possible interaction between converter residue 759 and relay residue 509 in the actin-detached conformation, which is lost in R759E but is restored in N509K/R759E. This detailed kinetic analysis of Drosophila myosin carrying the R759E mutation shows that the interface between the relay loop and converter domain is important for fine-tuning myosin kinetics, in particular ATP binding and hydrolysis.
Keywords: actin; fluorescence; homology modeling; kinetics; muscle; myosin; protein structure-function; sequence alignment.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


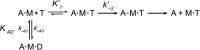





References
-
- O'Connell C. B., Tyska M. J., and Mooseker M. S. (2007) Myosin at work: motor adaptations for a variety of cellular functions. Biochim. Biophys. Acta 1773, 615–630 - PubMed
-
- Reggiani C., Bottinelli R., and Stienen G. J. (2000) Sarcomeric myosin isoforms: fine tuning of a molecular motor. News Physiol. Sci. 15, 26–33 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

