The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption
- PMID: 26588174
- PMCID: PMC4840470
- DOI: 10.1097/QAD.0000000000000953
The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption
Erratum in
-
The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption: Erratum.AIDS. 2016 Sep 10;30(14):2259. doi: 10.1097/01.aids.0000499516.66930.89. AIDS. 2016. PMID: 27579487 No abstract available.
Abstract
Objectives: Therapies to achieve sustained antiretroviral therapy-free HIV remission will require validation in analytic treatment interruption (ATI) trials. Identifying biomarkers that predict time to viral rebound could accelerate the development of such therapeutics.
Design: A pooled analysis of participants from six AIDS Clinical Trials Group ATI studies to identify predictors of viral rebound.
Methods: Cell-associated DNA (CA-DNA) and CA-RNA were quantified in pre-ATI peripheral blood mononuclear cell samples, and residual plasma viremia was measured using the single-copy assay.
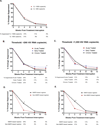
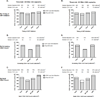
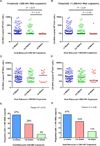
Results: Participants who initiated antiretroviral therapy (ART) during acute/early HIV infection and those on a non-nucleoside reverse transcriptase inhibitor-containing regimen had significantly delayed viral rebound. Participants who initiated ART during acute/early infection had lower levels of pre-ATI CA-RNA (acute/early vs. chronic-treated: median <92 vs. 156 HIV-1 RNA copies/10 CD4 cells, P < 0.01). Higher pre-ATI CA-RNA levels were significantly associated with shorter time to viral rebound (≤4 vs. 5-8 vs. >8 weeks: median 182 vs. 107 vs. <92 HIV-1 RNA copies/10 CD4 cells, Kruskal-Wallis P < 0.01). The proportion of participants with detectable plasma residual viremia prior to ATI was significantly higher among those with shorter time to viral rebound.
Conclusion: Higher levels of HIV expression while on ART are associated with shorter time to HIV rebound after treatment interruption. Quantification of the active HIV reservoir may provide a biomarker of efficacy for therapies that aim to achieve ART-free HIV remission.
Figures

Comment in
-
Unite forces to validate biomarkers in the quest for lasting HIV remission.AIDS. 2016 Jul 17;30(11):1859-60. doi: 10.1097/QAD.0000000000001118. AIDS. 2016. PMID: 27351931 No abstract available.
References
-
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. - PubMed
-
- Colven R, Harrington RD, Spach DH, Cohen CJ, Hooton TM. Retroviral rebound syndrome after cessation of suppressive antiretroviral therapy in three patients with chronic HIV infection. Ann Intern Med. 2000;133:430–434. - PubMed
-
- Bouldouyre MA, Charreau I, Marchou B, Tangre P, Katlama C, Morlat P, et al. Incidence and risk factors of thrombocytopenia in patients receiving intermittent antiretroviral therapy: a substudy of the ANRS 106-window trial. J Acquir Immune Defic Syndr. 2009;52:531–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI069501/AI/NIAID NIH HHS/United States
- AI36219/AI/NIAID NIH HHS/United States
- AI100699/AI/NIAID NIH HHS/United States
- R21 AI114448/AI/NIAID NIH HHS/United States
- 5P30AI060354-08/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069494/AI/NIAID NIH HHS/United States
- K08 AI100699/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

