High Spatiotemporal-Resolution Magnetic Tweezers: Calibration and Applications for DNA Dynamics
- PMID: 26588570
- PMCID: PMC4656881
- DOI: 10.1016/j.bpj.2015.10.018
High Spatiotemporal-Resolution Magnetic Tweezers: Calibration and Applications for DNA Dynamics
Abstract
The observation of biological processes at the molecular scale in real time requires high spatial and temporal resolution. Magnetic tweezers are straightforward to implement, free of radiation or photodamage, and provide ample multiplexing capability, but their spatiotemporal resolution has lagged behind that of other single-molecule manipulation techniques, notably optical tweezers and AFM. Here, we present, to our knowledge, a new high-resolution magnetic tweezers apparatus. We systematically characterize the achievable spatiotemporal resolution for both incoherent and coherent light sources, different types and sizes of beads, and different types and lengths of tethered molecules. Using a bright coherent laser source for illumination and tracking at 6 kHz, we resolve 3 Å steps with a 1 s period for surface-melted beads and 5 Å steps with a 0.5 s period for double-stranded-dsDNA-tethered beads, in good agreement with a model of stochastic bead motion in the magnetic tweezers. We demonstrate how this instrument can be used to monitor the opening and closing of a DNA hairpin on millisecond timescales in real time, together with attendant changes in the hairpin dynamics upon the addition of deoxythymidine triphosphate. Our approach opens up the possibility of observing biological events at submillisecond timescales with subnanometer resolution using camera-based detection.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

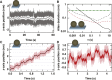
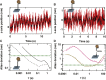


References
-
- Alberts B. Garland Science; New York: 2002. Molecular Biology of the Cell.
-
- Xie S.N. Single-molecule approach to enzymology. Single Mol. 2001;2:229–236.
-
- Dulin D., Lipfert J., Dekker N.H. Studying genomic processes at the single-molecule level: introducing the tools and applications. Nat. Rev. Genet. 2013;14:9–22. - PubMed
-
- Moffitt J.R., Chemla Y.R., Bustamante C. Recent advances in optical tweezers. Annu. Rev. Biochem. 2008;77:205–228. - PubMed
-
- Joo C., Balci H., Ha T. Advances in single-molecule fluorescence methods for molecular biology. Annu. Rev. Biochem. 2008;77:51–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

