A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes
- PMID: 26588783
- PMCID: PMC4654993
- DOI: 10.1016/j.immuni.2015.10.016
A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes
Abstract
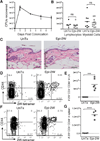
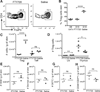
The skin is a site of constant dialog between the immune system and commensal bacteria. However, the molecular mechanisms that allow us to tolerate the presence of skin commensals without eliciting destructive inflammation are unknown. Using a model system to study the antigen-specific response to S. epidermidis, we demonstrated that skin colonization during a defined period of neonatal life was required for establishing immune tolerance to commensal microbes. This crucial window was characterized by an abrupt influx of highly activated regulatory T (Treg) cells into neonatal skin. Selective inhibition of this Treg cell wave completely abrogated tolerance. Thus, the host-commensal relationship in the skin relied on a unique Treg cell population that mediated tolerance to bacterial antigens during a defined developmental window. This suggests that the cutaneous microbiome composition in neonatal life is crucial in shaping adaptive immune responses to commensals, and disrupting these interactions might have enduring health implications.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




Comment in
-
"Bringing Up Baby" to Tolerate Germs.Immunity. 2015 Nov 17;43(5):842-4. doi: 10.1016/j.immuni.2015.10.020. Immunity. 2015. PMID: 26588777
-
Immune tolerance: A window of opportunity.Nat Rev Immunol. 2016 Jan;16(1):4. doi: 10.1038/nri.2015.6. Epub 2015 Nov 30. Nat Rev Immunol. 2016. PMID: 26616297 No abstract available.
References
-
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. - PubMed
-
- Allgaier H, Jung G, Werner RG, Schneider U, Zähner H. Epidermin: sequencing of a heterodetic tetracyclic 21-peptide amide antibiotic. Eur. J. Biochem. 1986;160:9–22. - PubMed
-
- Augustin J, Gotz F. Transformation of Staphylococcus epidermidis and other staphylococcal species with plasmid DNA by electroporation. FEMS Microbiology Letters. 1990;54:203–207. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- ImNIH/Intramural NIH HHS/United States
- K08 AR068409/AR/NIAMS NIH HHS/United States
- DP2-AR068130/AR/NIAMS NIH HHS/United States
- DP2 AR068130/AR/NIAMS NIH HHS/United States
- K08 AR062064/AR/NIAMS NIH HHS/United States
- K08-AR062064/AR/NIAMS NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- R01 AI107020/AI/NIAID NIH HHS/United States
- R21-AR066821/AR/NIAMS NIH HHS/United States
- 5P30CA082103-15/CA/NCI NIH HHS/United States
- U19 AI095261/AI/NIAID NIH HHS/United States
- R21 AR066821/AR/NIAMS NIH HHS/United States
- P30 CA082103/CA/NCI NIH HHS/United States
- P30 DK063720/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

