Molecular mechanism of positive allosteric modulation of the metabotropic glutamate receptor 2 by JNJ-46281222
- PMID: 26589404
- PMCID: PMC4728424
- DOI: 10.1111/bph.13390
Molecular mechanism of positive allosteric modulation of the metabotropic glutamate receptor 2 by JNJ-46281222
Abstract
Background and purpose: Allosteric modulation of the mGlu2 receptor is a potential strategy for treatment of various neurological and psychiatric disorders. Here, we describe the in vitro characterization of the mGlu2 positive allosteric modulator (PAM) JNJ-46281222 and its radiolabelled counterpart [(3) H]-JNJ-46281222. Using this novel tool, we also describe the allosteric effect of orthosteric glutamate binding and the presence of a bound G protein on PAM binding and use computational approaches to further investigate the binding mode.
Experimental approach: We have used radioligand binding studies, functional assays, site-directed mutagenesis, homology modelling and molecular dynamics to study the binding of JNJ-46281222.
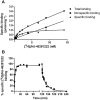
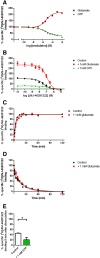
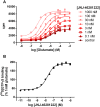
Key results: JNJ-46281222 is an mGlu2 -selective, highly potent PAM with nanomolar affinity (KD = 1.7 nM). Binding of [(3) H]-JNJ-46281222 was increased by the presence of glutamate and greatly reduced by the presence of GTP, indicating the preference for a G protein bound state of the receptor for PAM binding. Its allosteric binding site was visualized and analysed by a computational docking and molecular dynamics study. The simulations revealed amino acid movements in regions expected to be important for activation. The binding mode was supported by [(3) H]-JNJ-46281222 binding experiments on mutant receptors.
Conclusion and implications: Our results obtained with JNJ-46281222 in unlabelled and tritiated form further contribute to our understanding of mGlu2 allosteric modulation. The computational simulations and mutagenesis provide a plausible binding mode with indications of how the ligand permits allosteric activation. This study is therefore of interest for mGlu2 and class C receptor drug discovery.
© 2015 The British Pharmacological Society.
Figures


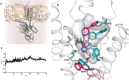

References
-
- Ball M, Boyd A, Churchill G, Cuthbert M, Drew M, Fielding M, et al (2012). Isoindolone formation via intramolecular Diels–Alder reaction. Org. Process Res. Dev. 16: 741–747.
-
- Cheng Y, Prusoff WH (1973). Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22: 3099–3108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

