MicroRNA-155 regulates host immune response to postviral bacterial pneumonia via IL-23/IL-17 pathway
- PMID: 26589478
- PMCID: PMC4773845
- DOI: 10.1152/ajplung.00224.2015
MicroRNA-155 regulates host immune response to postviral bacterial pneumonia via IL-23/IL-17 pathway
Abstract
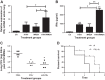
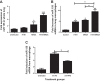
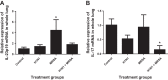
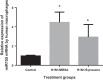
Postinfluenza bacterial pneumonia is associated with significant mortality and morbidity. MicroRNAs (miRNAs) are small, noncoding RNAs that regulate gene expression posttranscriptionally. miR-155 has recently emerged as a crucial regulator of innate immunity and inflammatory responses and is induced in macrophages during infection. We hypothesized upregulation of miR-155 inhibits IL-17 and increases susceptibility to secondary bacterial pneumonia. Mice were challenged with 100 plaque-forming units H1N1 intranasally and were infected with 10(7) colony-forming units of MRSA intratracheally at day 5 postviral challenge. Lungs were harvested 24 h later, and expression of miR-155, IL-17, and IL-23 was measured by real-time RT-PCR. Induction of miR-155 was 3.6-fold higher in dual-infected lungs compared with single infection. miR-155(-/-) mice were protected with significantly lower (4-fold) bacterial burden and no differences in viral load, associated with robust induction of IL-23 and IL-17 (2.2- and 4.8-fold, respectively) postsequential challenge with virus and bacteria, compared with WT mice. Treatment with miR-155 antagomir improved lung bacterial clearance by 4.2-fold compared with control antagomir postsequential infection with virus and bacteria. Moreover, lung macrophages collected from patients with postviral bacterial pneumonia also had upregulation of miR-155 expression compared with healthy controls, consistent with observations in our murine model. This is the first demonstration that cellular miRNAs regulate postinfluenza immune response to subsequent bacterial challenge by suppressing the IL-17 pathway in the lung. Our findings suggest that antagonizing certain microRNA might serve as a potential therapeutic strategy against secondary bacterial infection.
Keywords: IL-17; miR-155; postviral bacterial pneumonia.
Copyright © 2016 the American Physiological Society.
Figures




Similar articles
-
The role of IL-27 in susceptibility to post-influenza Staphylococcus aureus pneumonia.Respir Res. 2015 Feb 5;16(1):10. doi: 10.1186/s12931-015-0168-8. Respir Res. 2015. PMID: 25651926 Free PMC article.
-
CCR2 mediates increased susceptibility to post-H1N1 bacterial pneumonia by limiting dendritic cell induction of IL-17.Mucosal Immunol. 2019 Mar;12(2):518-530. doi: 10.1038/s41385-018-0106-4. Epub 2018 Nov 29. Mucosal Immunol. 2019. PMID: 30498200 Free PMC article.
-
Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice.J Immunol. 2011 Feb 1;186(3):1666-1674. doi: 10.4049/jimmunol.1002194. Epub 2010 Dec 22. J Immunol. 2011. PMID: 21178015 Free PMC article.
-
Postviral Complications: Bacterial Pneumonia.Clin Chest Med. 2017 Mar;38(1):127-138. doi: 10.1016/j.ccm.2016.11.006. Epub 2016 Dec 13. Clin Chest Med. 2017. PMID: 28159155 Free PMC article. Review.
-
Postinfluenza bacterial pneumonia: host defenses gone awry.J Interferon Cytokine Res. 2010 Sep;30(9):643-52. doi: 10.1089/jir.2010.0049. J Interferon Cytokine Res. 2010. PMID: 20726789 Free PMC article. Review.
Cited by
-
Extracellular Vesicle Associated Non-Coding RNAs in Lung Infections and Injury.Cells. 2021 Apr 21;10(5):965. doi: 10.3390/cells10050965. Cells. 2021. PMID: 33919158 Free PMC article. Review.
-
IL-17 in the lung: the good, the bad, and the ugly.Am J Physiol Lung Cell Mol Physiol. 2018 Jan 1;314(1):L6-L16. doi: 10.1152/ajplung.00344.2017. Epub 2017 Aug 31. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 28860146 Free PMC article. Review.
-
Interpreting the Function of the IL-23/IL-17 Axis through Bioinformatics.Endocr Metab Immune Disord Drug Targets. 2025;25(6):429-441. doi: 10.2174/0118715303316226240823045641. Endocr Metab Immune Disord Drug Targets. 2025. PMID: 39318016 Review.
-
Bacterial and viral infections and related inflammatory responses in chronic obstructive pulmonary disease.Ann Med. 2021 Dec;53(1):135-150. doi: 10.1080/07853890.2020.1831050. Ann Med. 2021. PMID: 32997525 Free PMC article. Review.
-
DsRNA induction of microRNA-155 disrupt tight junction barrier by modulating claudins.Asia Pac Allergy. 2020 Apr 27;10(2):e20. doi: 10.5415/apallergy.2020.10.e20. eCollection 2020 Apr. Asia Pac Allergy. 2020. PMID: 32411585 Free PMC article.
References
-
- ARDS Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA 307: 2526–2533, 2012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

